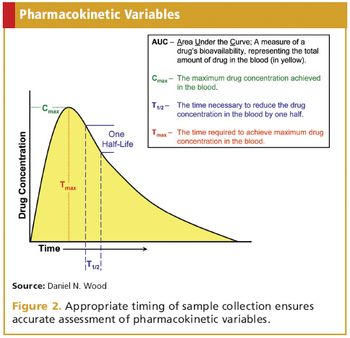
Guidance for the design and conduct of international bioequivalence trials.

Guidance for the design and conduct of international bioequivalence trials.

Taking advantage of the eCTD's cross-application feature, linking from NDA back to IND.

Pressure to reduce health care spending puts R&D, costs, and coverage on the negotiating table.

Contradicting decisions call for a unified clinical trial authorization process.
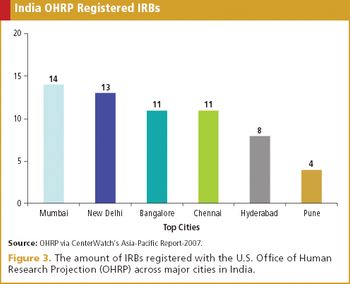
Understanding common myths and truths surrounding one of the fastest growing clinical trial markets.

A look at the Pocket Guide to the EU Directives for Clinical Research, published by the Institute of Clinical Research.

Out of the horrors of World War II came the Nuremberg Code, the prototype for human research protection.

European Union health ministers begin reviewing proposals to better adverse event assessment in Europe.
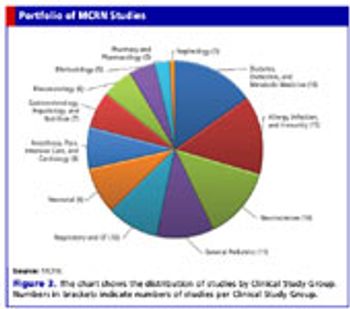
Inside the Medicines for Children Research Network and its work to improve medicines for the young.

FDA leaders stress innovation and disclosure in promoting the agency's public health mission.

Jill Wechsler provides a special report of a briefing with FDA's new commissioner Margaret Hamburg.

Session poses the question can we do better?

How FDA is responding to the demands of a global world, where agency-regulated products are in the trillions.

A current look at FDA's role in electronic medical records.
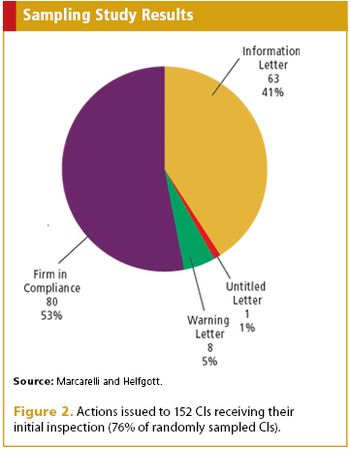
A probability sampling assessment by FDA takes a look at compliance in the medical device world.

EMEA releases new guideline for studies in PTSD, as new figures depict a lagging EU.
Roundtable participants discuss challenges and successes with early eCTD implementation.

What managers must know and do to overcome regulatory challenges and avoid hiccups.

Agency officials and sponsors anticipate stiffer oversight of research operations and disclosure requirements.

A look into how the European Union is handling the daunting health threats of the swine flu.

Critical comments of the drug evaluation process bring about strong reaction from industry.

Senior Manager of Global Regulatory Affairs for Wyeth discusses the implementation of eCTD and selecting the proper vendor.

Government funding slated to boost studies comparing medications to other treatments.

Changing rules on advanced therapy medicinal products to require more from clinical studies.

Shire Pharmaceuticals' Carol Rutkowski explains the benefits of training employees in regard to the regulatory eCTD submissions world.