The last mile in clinical research is integrating the research site to create a complete information supply chain.

The last mile in clinical research is integrating the research site to create a complete information supply chain.

Europe's clinical trial community has a lot to say about the EMEA's draft guidance on first studies in man.

The UK's revised model clinical trial agreements were launched, but have the changes saved time and money?

Expanded access to drugs for seniors has increased demand, focusing more attention on medical costs.

Getting past consent and ethical issues endemic in underserved populations to ensure quality GCP.

Individual countries hold the key to finding hot spots in growth regions like Central and Eastern Europe.

Guidelines and a checklist ensure those responsible for subject safety and data validity are doing their jobs.

Industry and patient groups embrace new rules but acknowledge concern over initiative's impact across EU.

Additional testing and monitoring requirements promise many changes for pharma R&D.

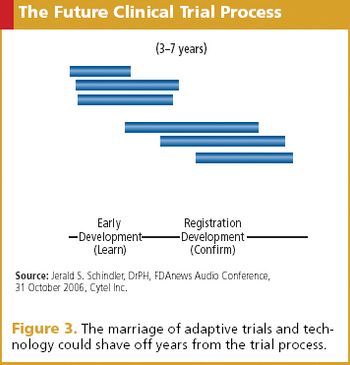
Until adaptive trials are the norm, industry needs to proceed with care and an iterative approach.

What Canada, the EU, and United States are doing to update their compassionate use regulations.

Journal editors and legislators expand the scope of clinical trial registration and results disclosure.

After a long and winding journey, now the real work begins on the EU Regulation for all those involved.
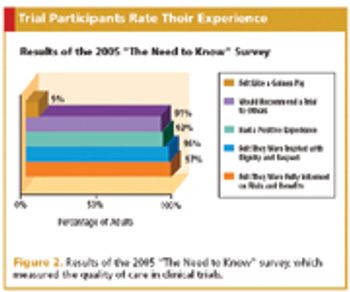
Who better to educate a wary public about clinical research than the industry's own?

New to the trials scene, many up-and-coming European firms are seeking the expertise of CROs they can trust.

New regulation provides the EU with a solid framework to stimulate R&D for children's medicines.

High-quality programs needed to protect subjects, improve data, harmonize global trials, and manage costs.

New report based on findings from a 2006 research project adds up savings using CDISC standards.

Added information to inform treatment decisions may drive up research costs for sponsors.

Five-module architecture organizes massive amounts of information included in a marketing submission.

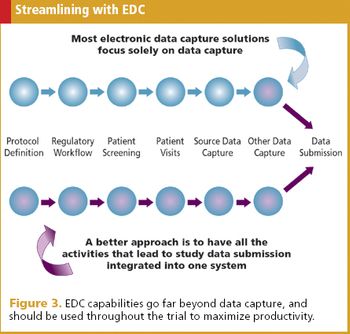
They're crucial in e-clinical applications, and thanks to new solutions, no longer out of reach for smaller companies.

Release of final Guidance is a step in the right direction for Agency and trials.

Group recommends ways to improve and increase clinical research in new report.
E-solutions are on the rise and gaining favor with FDA, as resistance finally gives way.

When it comes to readability, do as the Feds say and not as they do. Federal agencies may recommend that consent forms be written on a junior high school level, but it's a daunting task even for them.