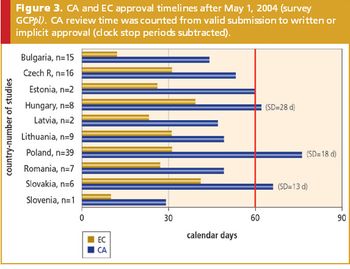
The impact of the EU Clinical Trials Directive on current clinical research practices

The impact of the EU Clinical Trials Directive on current clinical research practices

A system of checks and examinations that helps ensure the quality of clinical trials.

FDA seeks to reduce clinical research failures and make drug labeling more useful.
When negative results arise, novel analysis packages help speed the decision to move forward or pull the plug on a new drug.

As patents expire, clinical trials will be caught in the middle between copyists and original innovators.

Today's technology makes significant improvements in trial safety systems possible.

Drug safety and R&D issues will play a prominent role in user fee program revisions.
Changes prompt new oversight approaches by FDA and research institutions.

As some EU countries struggle with the Directive's regulatory demands, emerging markets such as China, India, and Russia continue to bolster their clinical research and drug development programs.

GRPs are not another set of guidelines or "additional hurdles" prepared by authorities.

New paper from the European Medicines Agency explores future vaccine guideline.

Political and technical tensions in the EU.

While veteran officials keep the agency running, changes continue at FDA's new drug review and safety oversight offices.

A comprehensive listing of U.S. departments and offices that includes the telephone numbers of directors, commissioners, and advisors.

Three recent initiatives address the reputation of the health care industry in the EU.
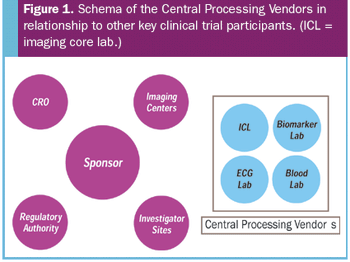
Technological advances have lead to an increasingly important role for centralized imaging laboratories.
Implementing ICH E14 to define cardiac safety in new drug development
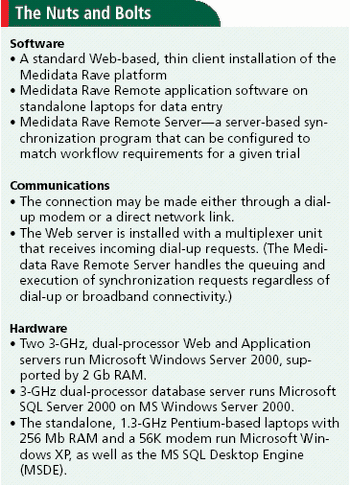
Janssen Pharmaceutica and Medidata Solutions join forces to institute eSourcing.

Clinical researchers strive to meet ongoing challenges posed by the EU Directive.

Parliament's first review of the new pediatric rules stirs controversy.

Curbs on consulting and financial interests aim to restore researchers' credibility.

Even with current trial management systems, steps can be taken right now to greatly increase efficiency.

As laudable as it may appear to be, the Critical Path Initiative will create as many problems as it solves.

The "Critical Path" is the scientific process through which a potential pharmaceutical, or medical device is transformed from a discovery into a medical product. Along the critical path, scientific tests and tools are used to predict whether a product candidate will be safe and effective, to assess how prototypes interact with the human body, and to guide the sponsor in choosing an appropriate dose and regimen or device size and/or placement. To bring a product to market successfully and efficiently, product sponsors need scientifically sound approaches.

Commissioner Crawford's challenge is to restore public confidence in drug safety and approvals.