
Slowdown in new drugs to market prompts antitrust investigation by EU competition authorities.

Slowdown in new drugs to market prompts antitrust investigation by EU competition authorities.

Global harmonization, regulatory flexibility promise to expand treatments for rare and neglected diseases.

Detailed charter defines the roles of the group and their relationships with other study team members-and it's available inside as a word document that you can download for use.

From a new guidance on inspections to a bid to help the EU regain its R&D prowess, the Agency is busy.

Comparative drug analysis aims to address costs and value as candidates eye curbs on drug spending.

Chris Bode, PhD, vice president of corporate development for Absorption Systems, explains the impact of in vitro models relative to the Critical Path Initiative.

In the European Union, regulators are agonizing over more than just clinical trials.
Low costs, a rich patient base, and strong talent pool characterize Africa's most populated country.

Survey uncovers current market and future directions for the European clinical trials industry.

How one group is transforming clinical cancer research across all of Europe.

With backing from pharma and high hopes, Eu takes steps to improve front-end approaches to R&D.

Ketek probe raises questions about research oversight by FDA, sponsors, and investigators.
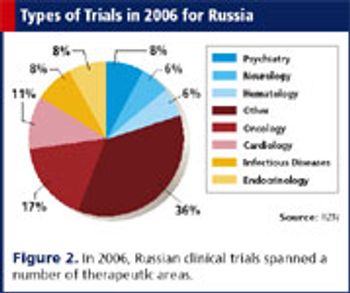
Since 1993, investments from big pharma have helped develop a solid infrastructure favorable to CROs.

New regulations prompt the EU to request more info from sponsors on clinical trial applications.

Paper is out and electronic is in at DIA's Electronic Document Management Conference.

FDA, NIH, and sponsors all struggle to implement complex registration and data access requirements.

Some of the tangible outcomes expected to emerge from a pediatric network include scientific and operational quality standards.

Truly integrated risk management requires breaking down silos and strong business leadership from the top.

The agency's 2008 budget fails to keep up with expanding programs, added safety concerns.

EMEA and EC admit to legislation difficulties, and many voice their ideas in an effort to make improvements.


FDA and sponsors implement FDAAA as pressure builds to curb drug prices and tweak the R&D process.

Lack of minority clinical investigators behind dearth of minority subjects in trials.


Along with the titles and phone numbers of personnel, View from Brussels columnist Peter O'Donnell gives an update on the regulatory front in Europe.