
FDA
Latest News

Latest Videos

More News

Pamela Tenaerts, MD, MBA, chief scientific officer, Medable; Luke Gelinas, PhD, senior chair director, Advarra; and Pam Diamond, MD, chief medical officer & co-founder, Curavit highlight the potential impact FDA's Diversity Action Plan guidance will have on industry.

Pamela Tenaerts, MD, MBA, chief scientific officer, Medable; Luke Gelinas, PhD, senior chair director, Advarra; and Pam Diamond, MD, chief medical officer & co-founder, Curavit share their initial thoughts on the announcement of FDA's Diversity Action Plan guidance.

In an interview with ACT editor Andy Studna at DIA 2024, Beakes-Read, head, global regulatory policy and intelligence, Johnson & Johnson Innovative Medicine highlights the integration of technological advancements into drug development and benefits patients are seeing from FDA's Accelerated Approval Program.

Johnson & Johnson is moving forward with a pair of Phase III trials of nipocalimab to reduce the risk of fetal neonatal alloimmune thrombocytopenia in alloimmunized pregnant patients.

Odronextamab is being evaluated for the treatment of relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma following two or more prior lines of systemic therapy.
Pivotal Phase III Study 302 trial data show an objective response rate of 36.2% based on an independent review committee assessment in the treatment of relapsed/refractory cutaneous T-cell lymphoma.
The FDA previously halted the IOV-LUN-202 trial following reports of a grade 5 serious adverse event that may have been linked to treatment with Iovance Biotherapeutics’ novel tumor infiltrating lymphocyte cell therapy LN-145, which is being evaluated in patients with advanced non–small cell lung cancer.
Epkinly (epcoritamab-bysp), subcutaneously administered, T-cell engaging, IgG1-bispecific antibody, was previously granted Breakthrough Therapy Designation for the treatment of patients with relapsed or refractory follicular lymphoma.
Trial data show Dupixent rapidly and significantly improved lung function compared to placebo in adults with uncontrolled chronic obstructive pulmonary disease with type 2 inflammation.
Ocifisertib has shown significant single-agent activity in both solid and liquid tumors.
The Phase I/II MajesTEC-1 trial (NCT03145181; NCT04557098) evaluated Tecvayli (teclistamab-cqyv) at a reduced dose of 1.5 mg/kg administered every two weeks in patients with relapsed/refractory multiple myeloma.
Data from the pivotal, global, randomized, double-blind, placebo-controlled, Phase III INDIGO trial show statistically significant and clinically meaningful progression-free survival for vorasidenib treating IDH-mutant gliomas.
Trial findings show Krazati (adagrasib) plus cetuximab was well tolerated with promising clinical activity among pretreated patients with locally advanced or metastatic colorectal cancer harboring a KRASG12C mutation
Datopotamab deruxtecan, the first TROP2-directed DXd antibody drug conjugate, is being evaluated for locally advanced or metastatic nonsquamous non-small cell lung cancer.
Results from the randomized, multicenter, open-label, Phase III FLAURA 2 trial show Tagrisso plus chemotherapy delayed disease progression by nearly nine additional months in adults with locally advanced or metastatic epidermal growth factor receptor-mutated non-small cell lung cancer.
Amtagvi (lifileucel) is a personalized, one-time therapeutic option for adult patients with unresectable or metastatic melanoma who received prior treatment with a PD-1 antibody, and in patients who are BRAF V600-positive, a BRAF inhibitor with or without a MEK inhibitor.

In clinical trials, Xolair was found to significantly increase the amount of peanuts, milk, egg, and cashew that it takes to trigger an allergic reaction in participants with food allergies.
Single-arm, open-label, multicenter, non-randomized, multicohort Phase II VISION trial (NCT02864992) of Tepmetko (tepotinib) for metastatic non–small cell lung cancer shows favorable objective response rate.

There were no safety or efficacy issues identified in the FDA's complete response letter for Lymphir, with no additional trials required.
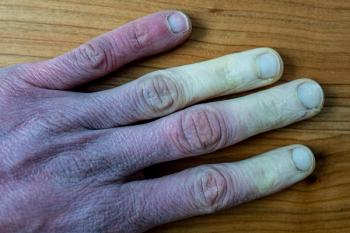
In clinical trials, patients administered Aurlumyn monotherapy experienced a significantly reduced risk of the need for amputation associated with severe frostbite.
Trials of Augtyro are ongoing for this tumor-agnostic indication, which could provide a durable treatment option for patients with NTRK-positive disease.
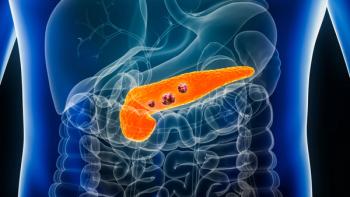
The Onivyde combination was evaluated in the open-label, multicenter, randomized Phase III NAPOLI 3 trial, showing significantly improved overall survival in patients with metastatic pancreatic adenocarcinoma.
Phase II trial data show positive survival results for BXCL701 combined with Keytruda (pembrolizumab) to treat patients with metastatic small cell neuroendocrine prostate cancer.
Phase IIb B-Clear trial shows bepirovirsen reduced HBsAg levels and HBV DNA following 24 weeks of treatment in patients with chronic hepatitis B.
Two multicenter, randomized, double-blind, placebo-controlled clinical trials showed that 53.1% of patients administered Eohilia achieved histologic remission compared with 1% of patients administered placebo.












