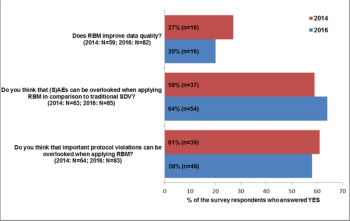
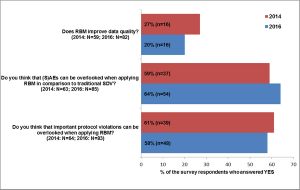
An RBM survey conducted in 2014 was repeated in 2016 in order to find out, how knowledge and practical experience with RBM have changed over that time period.

An RBM survey conducted in 2014 was repeated in 2016 in order to find out, how knowledge and practical experience with RBM have changed over that time period.
Published: October 20th 2017 | Updated: