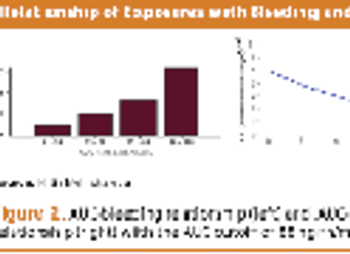
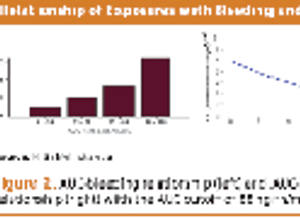
Collecting PK data in late phase trials led to alleviating additional trials, drug approval, and better dosing.

Collecting PK data in late phase trials led to alleviating additional trials, drug approval, and better dosing.
Published: February 1st 2012 | Updated: