
Continued embrace of precision dosing will reduce costs and optimize clinical outcomes.
CEO, Createrna Science and Technology, former Director of FDA’s Division of Pharmacometrics

Continued embrace of precision dosing will reduce costs and optimize clinical outcomes.
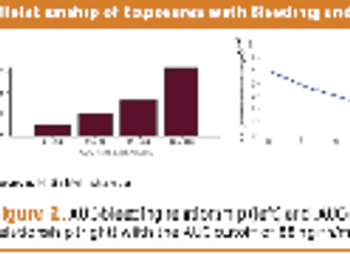
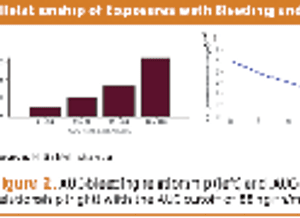
Collecting PK data in late phase trials led to alleviating additional trials, drug approval, and better dosing.
Published: February 1st 2012 | Updated: