Don Tracy, Associate Editor
Articles by Don Tracy, Associate Editor

In an interview with Applied Clinical Trials Associate Editor Don Tracy, Xandra Neuberger, associate director, regulatory affairs, PharmaLex, discusses new strategies that companies can implement to properly shift to the new EU clinical trial framework.

In an interview with Applied Clinical Trials Associate Editor Don Tracy, Xandra Neuberger, associate director, regulatory affairs, PharmaLex, offers her thoughts on what EU clinical trial regulations companies need to be the most aware of.

In an interview with Applied Clinical Trials Associate Editor Don Tracy, Xandra Neuberger, associate director, regulatory affairs, PharmaLex, discusses the goals of the EU clinical trial regulation.
An exploratory analysis from the TROPION-Lung01 Phase III trial found that tumors expressing TROP2 were associated with significantly improved responses to datopotamab deruxtecan compared to docetaxel in patients with non-small cell lung cancer.
Results from the Phase III MARIPOSA study found that the combination therapy showed an 8% improvement in overall survival in non-small cell lung cancer compared to osimertinib.

Study findings suggest that most qualitative systemic reviews implement a generic approach as opposed to focusing on equity, diversity, and inclusion issues.
According to a recent Phase II clinical trial conducted by Johns Hopkins Kimmel Cancer Center, all enrolled patients experienced a prostate-specific antigen reduction of 50% or more when treated with Olaparib.

Results from the joint Phase III clinical trial of the vaccine also demonstrated results comparable to the companies’ licensed COVID-19 vaccine.
Priority review of Imfinzi was based on promising results from the ADRIATIC Phase III trial in patients with limited-stage small cell lung cancer whose disease has not progressed following platinum-based concurrent chemoradiotherapy.
Results demonstrated prolonged progression free survival of two patients in Acclaim-1 study and partial remission from maintenance therapy in Acclaim-3 study.
Results from the Phase III MONeT clinical trial found that Abrysvo was well tolerated in adults over the age of 18 years old.

Results represent the first positive Phase III clinical trial in a dry eye chamber with a symptom as the primary endpoint.

Clinical trial for BI 3034701 is expected to evaluate the safety, tolerability, and pharmacokinetics in healthy men and overweight or obese individuals aged 18-55 years old.
The trial aims to address the limitations of first-generation COVID-19 vaccines in preventing infections and milder diseases.
Reportedly, data from the Vivacity-MG3 trial marks the first time an FcRn blocker demonstrated superiority over placebo in treated generalized myasthenia gravis (gMG).

Breakout session discusses the impact of incorporating decentralized elements into oncology clinical trials on data integrity, patient safety, and regulatory approvals.
The CAPItello-290 Phase III trial evaluated Truqap in combination with chemotherapy compared to placebo and chemotherapy for the treatment of locally advanced or metastatic triple-negative breast cancer.
Approval for Augtyro was based on results from the Phase I/II TRIDENT-1 trial, which demonstrated significant response rates in both tyrosine kinase inhibitor (TKI) naïve and TKI-pretreated patients.
Results of the trial found that monotherapy led to clinical benefit in 16.7% of patients, including complete and partial responses in renal cell carcinoma (RCC) patients.

Study results showed a 36% and 25% reduction in loss of asthma control (LOAC) with high and low doses of rilzabrutinib.
BTX-A51 aims to address GATA3 mutations, which affect around 15% of breast cancer patients.
Results from both trials demonstrated a significant reduction in annual asthma exacerbations over 52 weeks.

Results of the trial indicated that efsitora is non-inferior in A1C reduction compared to daily basal insulins.

A survey conducted at this year’s Pistoia Alliance conference determined that although 70% of life sciences experts acknowledge artificial intelligence’s potential, many still struggle with its initiation and implementation.

Partnership expected to utilize Walgreens’ pharmacies as recruitment and trial sites for a clinical study regarding obesity, weight issues, and type 2 diabetes, mainly in historically underrepresented groups.

Agency reports concerns amid an increase in submitted data that has been fabricated, duplicated, or unreliable from third-party laboratories.
Partnership comes amid two promising Phase III trial results for non-opioid, non-addictive medication for fibromyalgia.

A recent study showed that a multimodal machine learning approach accelerated the time in which investigators could determine the efficacy of sertraline treatment in major depressive disorder
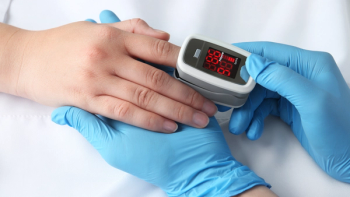
The MightySat Fingertip Pulse Oximeter is reportedly the first pulse oximeter available without a prescription.

Device designed to address tricuspid regurgitation through a vein in the leg.






























