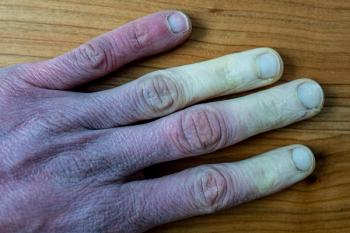
In clinical trials, patients administered Aurlumyn monotherapy experienced a significantly reduced risk of the need for amputation associated with severe frostbite.

In clinical trials, patients administered Aurlumyn monotherapy experienced a significantly reduced risk of the need for amputation associated with severe frostbite.

Breakout session at SCOPE Summit 2024 discusses the potential use of artificial intelligence to improve clinical trial strategy generation.
Samsung Biologics will provide antibody development and drug substance manufacturing services for LegoChem Biosciences' antibody-drug conjugate program with hopes of submitting an Investigational New Drug application to the FDA in 2025.
Study indicated that nipocalimab demonstrated significant improvements in the symptoms of generalized myasthenia gravis and Sjögren's disease.

In an interview with ACT Associate Editor Don Tracy, Shubh Goel, Head of Immuno-Oncology and Gastrointestinal Tumors, US Oncology Business Unit, AstraZeneca, provides her thoughts on the success of the trial.
Research collaboration between DELFI and Immunocore Holdings will investigate the potential of the DELFI-TF assay in predicting the efficacy of ImmTAC-based treatments early in the therapy process.
A pivotal Phase III clinical trial is currently evaluating treatment with rusfertide, a potential first-in-class treatment for polycythemia vera.

Tremfya achieved the co-primary endpoints of Scalp-Specific Investigator Global Assessment score of 0/1 and Psoriasis Scalp Severity Index 90 response at week 16 in the trial.
NK010 is a non-genetically modified natural killer cell therapy that dislayed significant potential in inhibiting strong tumor growth on ovarian cancers in animal studies.
Satri-cel (satricabtagene autoleucel) showed a promising safety and efficacy profile in patients with gastric or gastroesophageal cancer and pancreatic cancer.
Opdivo (nivolumab) with Yervoy (ipilimumab) improved progression-free survival in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer.

New machine learning technology combines artificial intelligence with automated experiments.
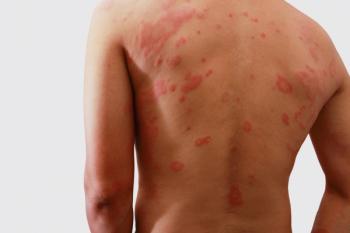
A pair of clinical trials show the rapid and reliable efficacy of Zoryve in treating atopic dermatitis and seborrheic dermatitis.
PrEPVacc halts study of experimental HIV vaccine regimens and a new form of oral pre-exposure prophylaxis.

DREAMM-7 was a Phase 3, multicenter, open-label, randomized trial analyzing the efficacy and safety of Blenrep plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously administered at least one prior line of treatment.

Study aims to find ways industry gets involved in the most influential clinical trials, and how transparent these trials are.

Medication focuses on the acute treatment of migraines.

Study emphasizes role in bridging research and development and commercial/marketing functions.

Findings suggests implementation could save time and money, but needs to be evaluated carefully.
Studies with Zenith-CKD showed significant albuminuria reduction.
BI 690517 is a novel, potent, highly selective aldosterone synthase inhibitor that reduces the progression of kidney damage and lowers the risk of cardiovascular events in patients with chronic kidney disease.

SLS009 is a novel CDK9 inhibitor under investigation for the treatment of relapsed/refractory peripheral T-cell lymphomas.

Furmonertinib is in development for the treatment of advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations.

Breakout session challenges current creation methods.

Steve Rosenberg, CEO, uMotif, discusses how patients would like to be involved in clinical trials as innovations in digital technologies continue to evolve.

Session discusses next steps to advancing evidence generation systems.

SCOPE session discusses key challenges of evaluating potential providers.

Panel at SCOPE discusses applying novel evidence in regulatory decision-making.

Panel at SCOPE discusses the possibilities of research in a post-COVID world.