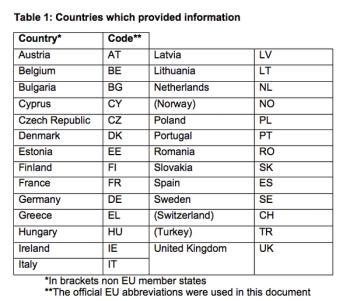
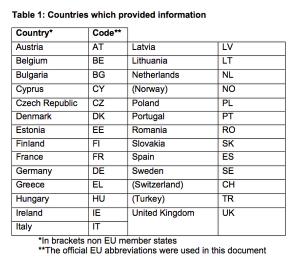
The Pediatric Working Group of EUCROF launched an initiative to achieve standardization of generic Informed Consent Form and Assent Form templates within European countries. The differences are noted based on a prior survey.

The Pediatric Working Group of EUCROF launched an initiative to achieve standardization of generic Informed Consent Form and Assent Form templates within European countries. The differences are noted based on a prior survey.
Published: October 5th 2016 | Updated: