The Pediatric Working Group of EUCROF launched an initiative to achieve standardization of generic Informed Consent Form and Assent Form templates within European countries. The differences are noted based on a prior survey.

The Pediatric Working Group of EUCROF launched an initiative to achieve standardization of generic Informed Consent Form and Assent Form templates within European countries. The differences are noted based on a prior survey.
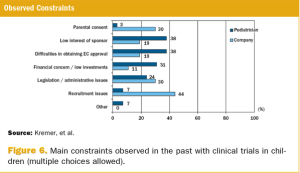
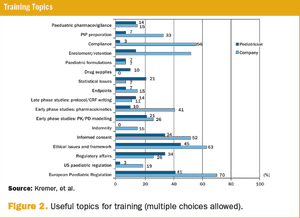
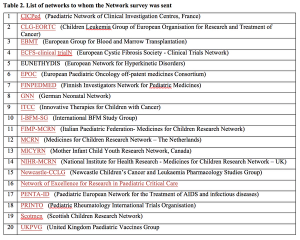
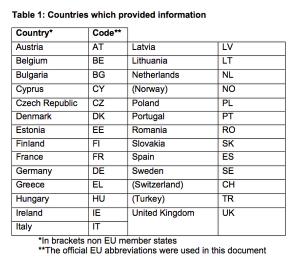
Pair of surveys provide a rare formal peek into the relationship between sponsors and pediatric clinical research networks. A lack of awareness, incongruent expectations, and insufficient communication have hindered collaboration between the two parties. Developing standardized procedures and better information exchange could help strengthen the connection going forward.
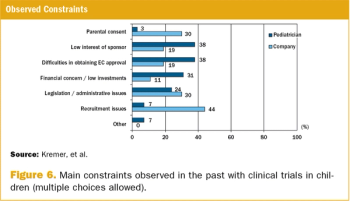
The Pediatric Working Group of the European CRO Federation conducted a follow-up survey on the status of pediatric clinical trials in Europe.
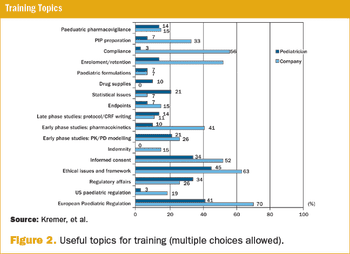
A survey on the perception of European pediatricians and industry/CROs
Published: February 25th 2014 | Updated:
Published: February 1st 2014 | Updated:
Published: January 8th 2016 | Updated:
Published: October 5th 2016 | Updated: