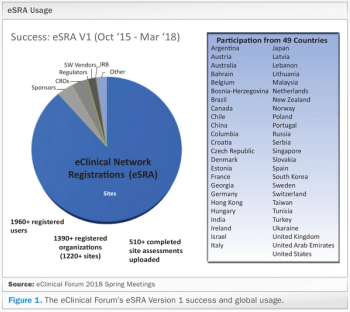
An increase in use of EHR systems at clinical investigative sites adds more expectations for clinical trial sponsors to comply by regulatory requirements.

An increase in use of EHR systems at clinical investigative sites adds more expectations for clinical trial sponsors to comply by regulatory requirements.
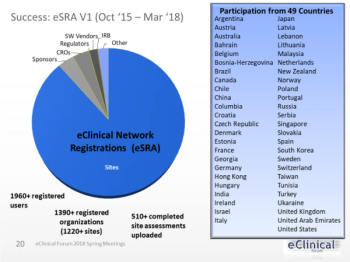
Electronic Health Record (EHR) systems are being used increasingly by clinical investigative sites.
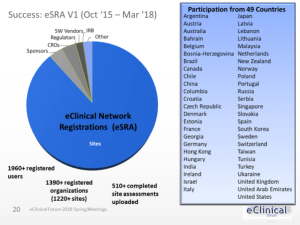
Published: December 13th 2018 | Updated:
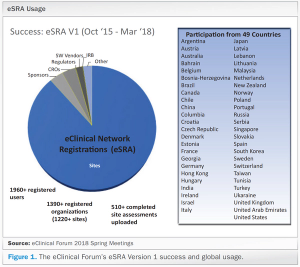
Published: January 1st 2019 | Updated: