
Kenneth G. Faulkner, PhD
Advertisement
Articles by Kenneth G. Faulkner, PhD


While not all clinical trial assessments can be done virtually, there are many that can be adapted for virtual use.
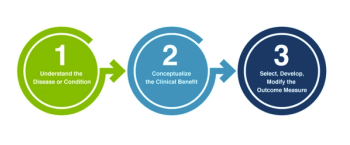
The four new methodological Patient-Focused Drug Development guidance documents the FDA is currently developing for the industry that incorporate patient experience data into drug development, summarized.
Advertisement
Latest Updated Articles
 Decentralized vs Digital Clinical Trials: There is a Difference
Decentralized vs Digital Clinical Trials: There is a DifferencePublished: November 12th 2021 | Updated:
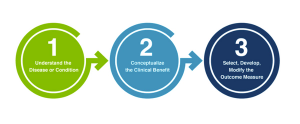 FDA Patient-Focused Drug Development Guidance Update: Incorporating Patient Experience Data in Clinical Trials
FDA Patient-Focused Drug Development Guidance Update: Incorporating Patient Experience Data in Clinical TrialsPublished: May 28th 2020 | Updated:
Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
2
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
3
Accelerate Clinical Trials with AI-Enhanced Financial Management
4
SCOPE Summit 2026 Keynote Fireside Chat: Aligning Purpose, Innovation, and Operational Excellence in Clinical Development
5