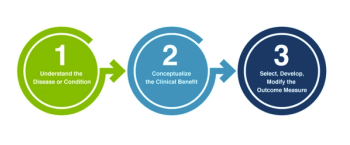
The four new methodological Patient-Focused Drug Development guidance documents the FDA is currently developing for the industry that incorporate patient experience data into drug development, summarized.

The four new methodological Patient-Focused Drug Development guidance documents the FDA is currently developing for the industry that incorporate patient experience data into drug development, summarized.
Published: May 28th 2020 | Updated: