
Applied Clinical Trials

Applied Clinical Trials

Applied Clinical Trials
The "discussion draft" for legislation to speed "21st Century Cures" to patients emerged very quietly on Capitol Hill recently, muted by an absence of bipartisan support which had generated considerable enthusiasm for this effort to promote biomedical research and streamline regulation.

Applied Clinical Trials
Efforts are escalating to encourage sponsors, research institutions, and clinical investigators to accept oversight for multi-center studies by central institutional review boards (IRBs), as seen in several discussions of this topic at the December conference on "Advancing Ethical Research" sponsored by Public Responsibility in Medicine & Research.

Applied Clinical Trials
In early January, Novartis selected Qualcomm Life as a global digital health collaborator for its Trials of The Future program.

Applied Clinical Trials
The World Health Report 2013 argues that universal health coverage cannot be achieved without the evidence from scientific research, and in order to better manage healthcare, clinical research needs to keep up-to-date and advance in line with society in general.
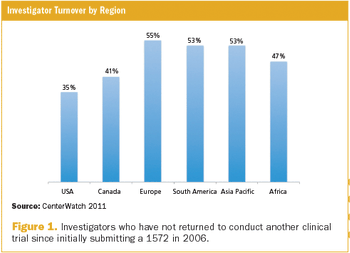
Applied Clinical Trials
The sharp rise in ongoing clinical research studies is driving demand for greater participation in research by physicians as well as by patients.1

Applied Clinical Trials
In the healthcare arena, the concept of patient-centeredness has expanded over the last 50 years, beginning as a term to describe patient engagement in self-health management and evolving to include various aspects of patient engagement in healthcare research.1
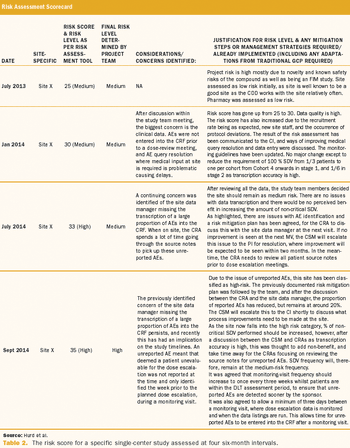
Applied Clinical Trials
Cancer Research UK (CRUK) followed such advice when it decided to have its Center for Drug Development (CDD) adopt a risk-based monitoring (RBM) approach across its entire portfolio of clinical trials.

Applied Clinical Trials
Sponsor companies face intense pressure to deliver higher levels of efficiency and drug development performance. A growing number of sponsors are now acting on the belief that improvements in protocol design feasibility hold the key to addressing and easing some of these pressures.
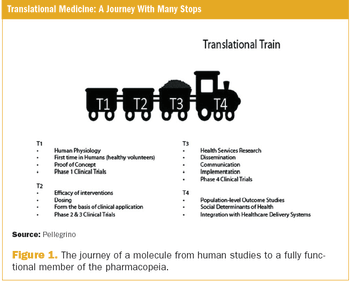
Applied Clinical Trials
The organization of healthcare is changing rapidly. The healthcare delivery system is increasingly powered by payers and regulators, and this directs both clinical medicine and drug development. Partly because of this change, the drug development process has been heavily scrutinized, and a great emphasis has been placed on more efficient translation of basic science into useful medicines.

Applied Clinical Trials
A surge in review activity at the FDA in December resulted in a near-record approval of 41 new drugs and biologics last year, the most since a record 56 approvals in 1996.
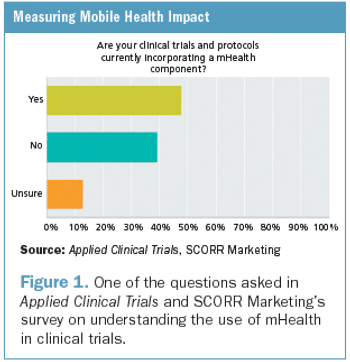
Applied Clinical Trials
After a five-year review of clinical trial quality measurement research and practices, we have concluded that clinical trial quality measurement does not meet current scientific standards

Applied Clinical Trials
Agency proposes new standards in hopes of capitalizing on 2014 surge in new drug approvals.

Applied Clinical Trials
The five-year figure doesn't always determine which cancer types are most commonly being studied in late-phase trials.

Applied Clinical Trials
While such discussions have advanced in Europe, two key omissions from the dialogue may limit any real changes.

Applied Clinical Trials
Sites: Integrated Clinical Research Boosting the Investigator Pool Trial Design: Remote Patient-Centered Studies Risk-Based Monitoring in Action Also in this issue: New Drug Approval Momentum mHealth in Clinical Trials Simplifying Protocol Design