
Applied Clinical Trials
Addressing the health needs of host nations while guarding against subject exploitation and other pitfalls.

Applied Clinical Trials
Addressing the health needs of host nations while guarding against subject exploitation and other pitfalls.
Applied Clinical Trials
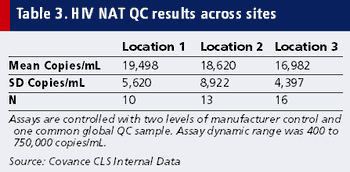
Achieving quality viral biomarker data from around the globe that measure up to standard protocol requirements

Applied Clinical Trials
For truly informed consent, methods should gauge a subject's real grasp of a clinical trial's complexities.

Applied Clinical Trials
A high-quality medical infrastructure and low costs make South Africa--largely an untapped opportunity--an attractive alternative for conducting clinical trials.

Applied Clinical Trials
Adequate female representation is mandatory, but gender differences should not be overestimated.

Applied Clinical Trials
Based on the numbers, sponsors will rapidly change their clinical trials midstream.

Applied Clinical Trials
A groundbreaking article from the last decade is just as applicable today.

Applied Clinical Trials
Clinical departments are very expensive to run and maintain.

Applied Clinical Trials
Regulators and researchers seek to curb the overload on IRBs and harmonize AER policies.

Applied Clinical Trials
For the EU, these new good clinical practice rules come not from Brussels, but Mt. Sinai.
Applied Clinical Trials
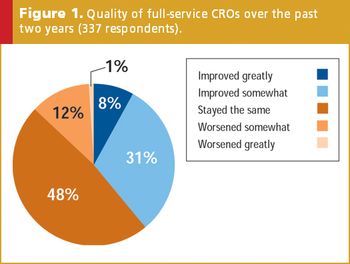
Following more than two decades of growing involvement in nearly all aspects of drug development, full-service and niche-services contract research organizations (CROs) have become a common and integral part of most sponsor companies' clinical research teams.

Applied Clinical Trials
What you need to know to save the bottom line...and your sanity.
Applied Clinical Trials
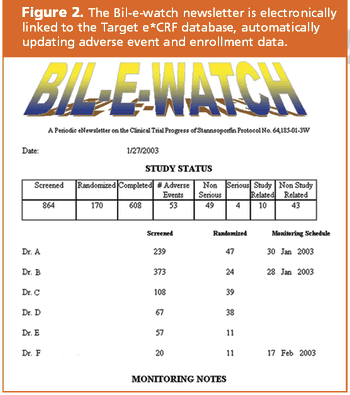
Target Health and Infacare used a CRF database to answer all of FDA's questions.