
Applied Clinical Trials
Using predictive modeling to combat the challenges of study enrollment.

Applied Clinical Trials
Using predictive modeling to combat the challenges of study enrollment.

Applied Clinical Trials
Thought leaders in the subject recruitment field come together to discuss current issues and potential solutions.

Applied Clinical Trials
Hear from the first FDA liaison in the Office of International Programs.
Applied Clinical Trials
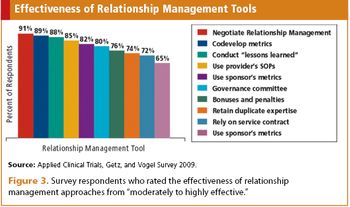
New survey captures sponsors changing global usage of, and relationships with, CRO partners.
Applied Clinical Trials
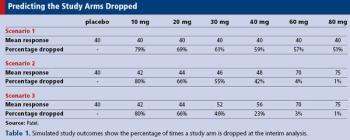
How simulation can help in the planning and implementation of adaptive clinical trials.
Applied Clinical Trials
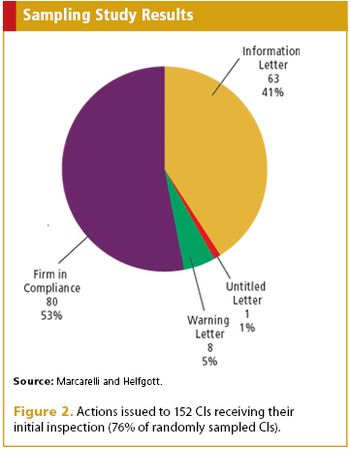
A probability sampling assessment by FDA takes a look at compliance in the medical device world.

Applied Clinical Trials
The European intiative of involving elderly in clinical trials picks up the pace as challenges are addressed.

Applied Clinical Trials
Undertaking clinical studies in this emerging region requires first overcoming natural barriers.
Applied Clinical Trials
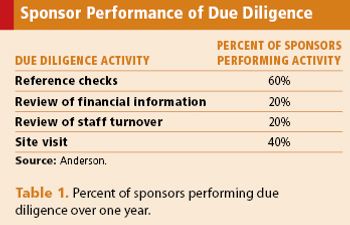
Knowing what you want from a CROs proposal will help make the most of the selection process.

Applied Clinical Trials
EMEA releases new guideline for studies in PTSD, as new figures depict a lagging EU.

Applied Clinical Trials
The latest eclinical software in the clinical trials industry.

Applied Clinical Trials
Learn what Applied Clinical Trial's readers told us in our annual reader's survey.

Applied Clinical Trials
Highlights and insights from a recent gathering of thousands of health care professionals.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.
Applied Clinical Trials
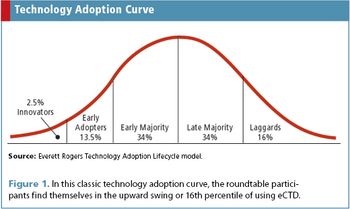
Roundtable participants discuss challenges and successes with early eCTD implementation.

Applied Clinical Trials
What managers must know and do to overcome regulatory challenges and avoid hiccups.

Applied Clinical Trials
Agency officials and sponsors anticipate stiffer oversight of research operations and disclosure requirements.