
Applied Clinical Trials
A new study by Premier Research shows that both American and European pharmaceutical companies are facing similar problems in regards to pediatric trial regulations.

Applied Clinical Trials
A new study by Premier Research shows that both American and European pharmaceutical companies are facing similar problems in regards to pediatric trial regulations.

Applied Clinical Trials
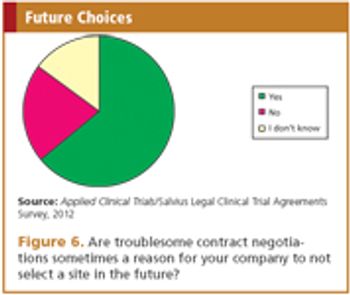
There are opportunities to make the negotiation process more efficient and reduce timelines.

Applied Clinical Trials
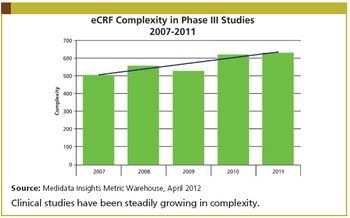
We collect too much data, burdening investigators, monitors, and data managers.

Applied Clinical Trials
European Parliament voted against clearing the agency's accounts because of management concerns.

Applied Clinical Trials
What works when and for whom in the era of comparative effectiveness research.

Applied Clinical Trials
An efficient system can be the difference between a drug being withdrawn or delivered to market.
Applied Clinical Trials
Medidata

Applied Clinical Trials
In clinical trials, the need for globally accessible toll-free and other phone numbers for trial functions is vital.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
The European Forum for GCP (EFGCP) is determined to achieve significant progress involving the elderly more closely in clinical research.

Applied Clinical Trials
Multiple proposals for streamlining research emerge in legislation, expert reports.

Applied Clinical Trials
A well designed CTCP can keep everyone motivated and focused on achieving trial objectives.

Applied Clinical Trials
CRO/Sponsor: Comparative Effectiveness Research and Precision Healthcare Pharmacovigilance Outsourcing: A Guide Survey: Negotiating a Clinical Trial Agreement Also in this issue: Trial Modernization EMA Under Attack Too Much Data? Community Portals