
Applied Clinical Trials
Agency concerned about how proposals in "Cures" bill will be crafted and implemented.

Applied Clinical Trials
Agency concerned about how proposals in "Cures" bill will be crafted and implemented.

Applied Clinical Trials
With patents for many NBCDs soon to expire, the need for regulatory guidance regarding follow-on versions of these products is crucial.

Applied Clinical Trials
The tone of agency’s annual report contrasts its recent candid stance on regulatory vision for Europe

Applied Clinical Trials
Applied Clinical Trials
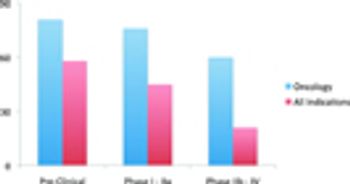
Study finds that almost 75% of cancer drug candidates rely on biomarker data.

Applied Clinical Trials
Study takes rare look at the financial and resource burden for sites in managing regulatory compliance.
Applied Clinical Trials
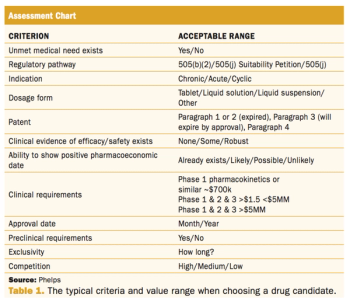
Understanding the benefits of this application route in helping companies withstand the hit of patent expiries.

Applied Clinical Trials
How the application of evolving M&S models are transforming full-research design strategies.
Applied Clinical Trials
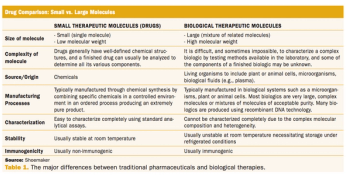
A regulatory perspective on the current state of protein science and the implications for biosimilar approval.


Applied Clinical Trials
Regulatory: Solving Generic Cliff with 505(b)(2) The U.S. Biosimilar Pathway Trial Design: Modeling & Simulation Strategies Lifecycle Management: Bridging Economic & Clinical Value Also in this issue: EMA's Dueling Tones on Regulatory Vision Regulatory Compliance: The Site Burden Assessing Safety for Follow-on NBCDs