Applied Clinical Trials
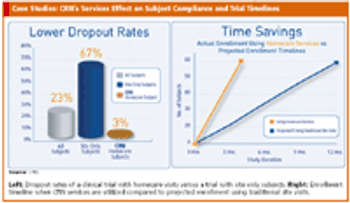
Specialty at-home and alternate-site testing services offer solution for subject recruitment issues.

Applied Clinical Trials
Specialty at-home and alternate-site testing services offer solution for subject recruitment issues.
Applied Clinical Trials
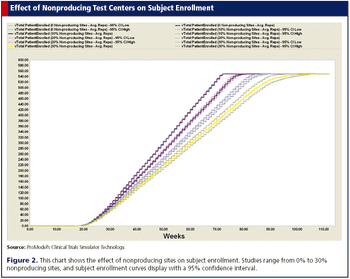
The use of computer simulation models to improve both site selection and subject recruitment.

Applied Clinical Trials
FDA is conducting more inspections to ensure that foreign clinical research meets GCP standards.
Applied Clinical Trials
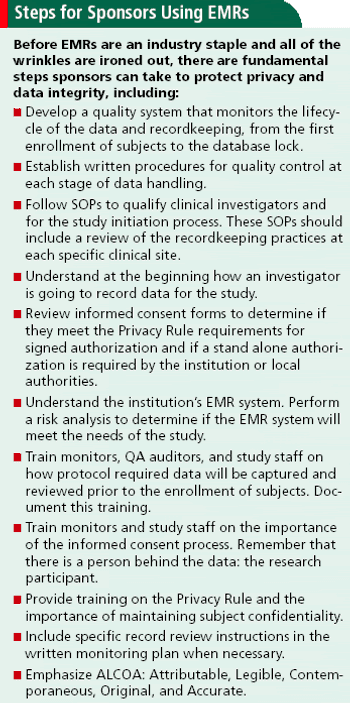
Shedding light on common EMR concerns about privacy, government regulations, and data integrity.

Applied Clinical Trials
The public considers clinical research important, but they're not fans of the people behind it.

Applied Clinical Trials
Health Market Science (King of Prussia, PA) is offering a new data-driven approach to site selection for clinical trials. With nine years experience under its belt with its physician masterfile and claims data
Applied Clinical Trials
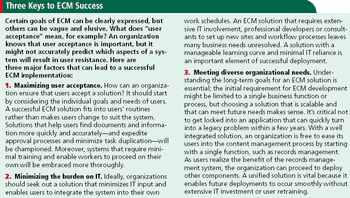
True efficiency requires the move to a company-wide ECM solution that supports the entire content lifecycle.

Applied Clinical Trials
A perspective on the agency's updated guidance on the use of computer systems in clinical research.
Applied Clinical Trials
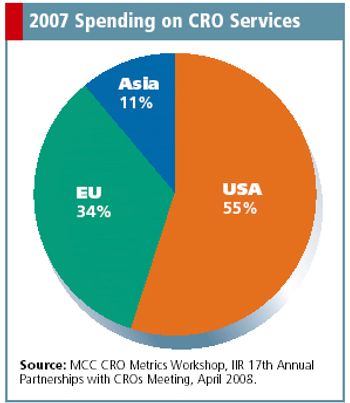
Connecting U.S. sponsors and foreign CROs in an increasingly outsourced market.

Applied Clinical Trials
Keeping score of Europe's one-year-old initiative to promote research for the pediatric community.

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
New technology has led to a transition from pens to digital signatures and it is becoming a part of everyday life for the pharmaceutical industry.

Applied Clinical Trials
Finding new ways to design and conduct clinical trials in order to prevent Phase III failures.