
Applied Clinical Trials
CRO/SPONSOR : Outsourcing to China REGULATORY : Warning Letter Analysis CRO/SPONSOR : Customer Expectation Management Also in this issue : EU Pediatric Regulation, Healthcare Data Informs Research, Modeling and Simulation

Applied Clinical Trials
CRO/SPONSOR : Outsourcing to China REGULATORY : Warning Letter Analysis CRO/SPONSOR : Customer Expectation Management Also in this issue : EU Pediatric Regulation, Healthcare Data Informs Research, Modeling and Simulation

Applied Clinical Trials
Industry news focusing on the people and organizations who work in the clinical trials profession.

Applied Clinical Trials
The new digital technologies used in clinical trials raise important ethical issues that warrant serious investigation, according to the European Forum for GCP (EFGCP), whose special 20th anniversary congress-to be held in Brussels, Belgium, in January.
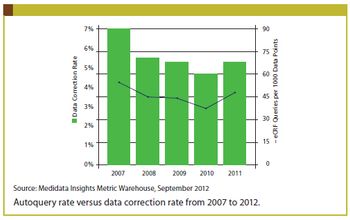
Applied Clinical Trials
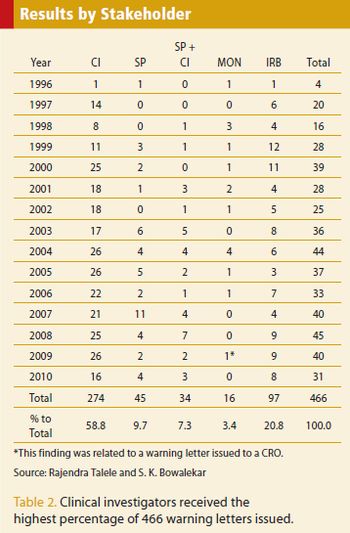
An analysis of warning letters and the findings reported from 1996 to 2010.

Applied Clinical Trials
To fully utilize the great advances in understanding the genomic basis of disease, clinicians need medical diagnostics able to identify those individuals most likely to benefit from pharmaceutical therapy, and those at risk for adverse events.

Applied Clinical Trials
What will it take for healthcare data to regularly inform research?

Applied Clinical Trials
Global reach for at-home study visits can accelerate clinical trials, says CEO.

Applied Clinical Trials
Building a culture of service excellence between sponsor/CROs and investigative sites.

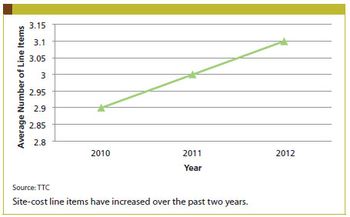

Applied Clinical Trials
Last October, Outcome Sciences announced its collaboration with the National Football League to update its Injury Surveillance System (ISS), since then, the pilot has been completed during the course of the 2011 NFL season and rolled out to all the teams for 2012.


Applied Clinical Trials
Conversion from paper to ePRO for an instrument designed to assess diabetes' impact on quality of life.

Applied Clinical Trials
Current trends in oncology trial terminations as analyzed by Citeline have revealed that sponsors are learning from the past and making gains in this troubled area of drug development.

Applied Clinical Trials
Why should sponsors and CROs consider using mobile nursing for clinical trials?

Applied Clinical Trials
Modeling and simulation has been heralded for some time as a possible answer to the industry's woes.

Applied Clinical Trials
The European Union is preparing a report on the lessons learned from its 2006 pediatric regulation.