
Applied Clinical Trials
While the future of the industry is still uncertain, a slew of uplifting ideas around innovation could help turn things around.

Applied Clinical Trials
While the future of the industry is still uncertain, a slew of uplifting ideas around innovation could help turn things around.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials November 2019 issue in an interactive PDF format.

Applied Clinical Trials
Exploring the nation’s opportunities for growth and the initiatives undertaken to build an attractive clinical trials ecosystem for early stage research.

Applied Clinical Trials
Roundtable gathers industry experts to discuss the guidance update’s early implementation and the shift toward risk-based study execution approaches.

Applied Clinical Trials
A compilation of recently released news briefs that pertain to the clinical trials industry.
Applied Clinical Trials
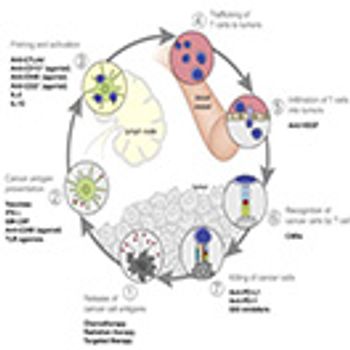
Outlining the three critical challenges that need to be addressed to make personalized cancer treatment a reality.

Applied Clinical Trials
Additional policies for registering clinical studies in U.S. and Europe aim to expand access to research data and provide timely information for patients on promising, new treatments.

Applied Clinical Trials
David Freeman, general manager, Information Ventures, for Quest Diagnostics, talks about the company's use of lab data to create an effective partnership for pharma and biotech companies.

Applied Clinical Trials
As China’s pharma industry continues to evolve and mature, it is hoped overseas expansion and greater innovation will be exported to serve the interests of patients.

Applied Clinical Trials
A shift in emphasis in healthcare strategy could reduce attention and funding to therapy and impose tougher controls over research projects, or possible mean a boost for innovative healthcare. A look at where these trends may go.