
Applied Clinical Trials
The shift from paper to cloud-based regulatory information management systems (RIMS) to address operations challenges.

Applied Clinical Trials
The shift from paper to cloud-based regulatory information management systems (RIMS) to address operations challenges.

Applied Clinical Trials
A compilation of recently released news briefs that pertain to the clinical trials industry.

Applied Clinical Trials
How one biotech is tackling the unmet need for additional immunotherapies and combination approaches in cancer.

Applied Clinical Trials
A new era for clinical trials supply compliance in the EU is almost here.

Applied Clinical Trials
The long-running SEND initiative has fostered new FDA-industry partnerships, opening the door to dramatic changes in toxicology data analysis.
Applied Clinical Trials
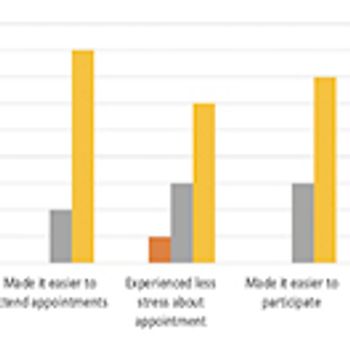
Recruitment study tests the use of a ride-sharing service.

Applied Clinical Trials
Click the title above to open the Applied Clinical Trials December 2019 issue in an interactive PDF format.

Applied Clinical Trials
Some thoughts around the industry continuing to grapple with the high price tag associated with drug development costs.

Applied Clinical Trials
Outlining the nation’s key changes and current requirements in processing investigational and new drug applications.

Applied Clinical Trials
Why and how FDA is funding a digital biomarkers study that centers on quality of life for patients with heart failure.

Applied Clinical Trials
A view of the notable policy strategies advanced in 2019 to boost drug development and review.

Applied Clinical Trials
Peter O’Donnell explores where the EU stands today in R&D pursuits for new antibacterial therapies.

Applied Clinical Trials
The regulatory path for virtual studies is nebulous and potentially difficult to navigate-finding a way forward requires a thorough understanding of the terrain and how to apply existing legal frameworks.

Applied Clinical Trials
Examining the unique aspects involved in preparing and submitting marketing applications for proposed treatments for rare disease.

Applied Clinical Trials
Higher screen failure and patient dropout rates are raising the imperative to pilot and implement new study conduct models in trial recruitment and retention.