
News







Regulatory enforcement actions, policy updates, and new guidelines show that ensuring the reliability of clinical data is an ongoing priority.



With more than 174,000 new cases expected to be diagnosed this year, explore emerging areas and future challenges prostate cancer treatment is facing.
Erin Finot, Global Head of Immuno-Oncology at IQVIA Biotech, discusses the immense challenges to overcome before advanced therapies become more widespread.





Michael Keens, COO of Firma Clinical, explores the beginning of patient centricity, common misconceptions related to its implementation, and offers steps to improve the ability to achieve patient centricity within drug development.
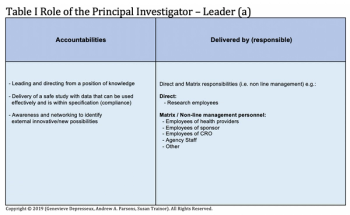
Taking a closer look at the skills expected from the Principal Investigator beyond the expected technical skills.

Executives from across the clinical research enterprise converge to discuss the steps-and technology-needed to strengthen industrywide data sharing.

Joel B. Selzer, co-founder and CEO, ArcheMedX, Inc., discusses the impact that healthcare technology has on clinical trials, focusing on the development of the company’s workforce readiness platform.

After years of siloed focus-and slow study start-ups-the U.S. Veterans Affairs agency embarks on implementing its multi-year initiative to bolster clinical research.

The two-year inquiry into possible maladministration at the EMA has intensified.

Click the title above to open the Applied Clinical Trials September 2019 issue in an interactive PDF format.

FDA and other regulators are responding with support for more flexible monitoring of clinical investigators and review of study records in order to limit study monitoring to certain situations.

Bristol-Myers Squibb’s cardiovascular development leader discusses the importance of diversifying clinical trials for better patient outcomes.

A view of highlights and trends from the September 2019 issue, alongside trends mentioned around site practices during the year.

Interest in benchmark data on the scope, performance, and economics of rare disease drug development efforts has grown.

Organizations that continue to disregard the technological needs of one of the industry’s core audiences run the risk of having their trials ignored.

Dr. Daniel Alkon, President & Chief Science Officer at Neurotrope, discusses novel approaches to AD therapy development and why his company chose to target patients with advanced AD for their own.

Global experts collaborate to form the Addressing Lupus Pillars for Health Advancement (ALPHA) Project in order to combat challenges in lupus drug development.
