
News









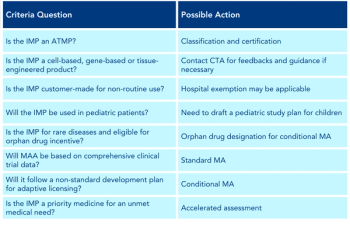
Dave Li explores the current information on the commercialization of advanced therapy medicinal products.



Clinical research must deploy innovative, disruptive approaches without disrupting patients.
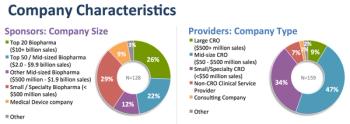
Findings from analyzed data from approximately 300 web survey responses representing over 200 global individual sponsor and provider organizations

Peter O'Donnell discusses the outpouring of support for Stella Kyriakides, who has been designated to lead European Union health policy for the next five years, and how she might need every bit of it.






Dr. Steven Fox, CEO of Akelos Inc., discusses the challenges of developing new non-opioid medical products as the need intensifies as the crisis worsens.







Simon Jones and Gordan Alexander, both of Prisym ID, explore the added complexity to the labeling of trial supplies that new regulations to EU 536/2014 will bring, specifically Annex VI.
