
The latest happenings in the clinical trials industry, all in one place.








Executive Medical Director of Oncology for Premier Research, Emile Youssef, discusses crucial challenges that must be addressed in order to fulfill the promise of precision oncology.





BioXcel Therapeutics Executives, Vimal Mehta and Vince O’Neill, discuss how they are actively using artificial intelligence to discover advanced therapies in CNS and oncology.



The need to see real-time study performance is vital, as teams focus on meeting timelines and ensuring resources are appropriately allocated, writes EVP of WCG, Jonathan Zung.

Peter O’Donnell writes of the support of ‘disruptive’ change in European health systems from European health professionals and policymakers from a recent gathering.



David Freeman, General Manager, Information Ventures, for Quest Diagnostics, discusses how his company can be an effective partner for pharma and biotech firms.
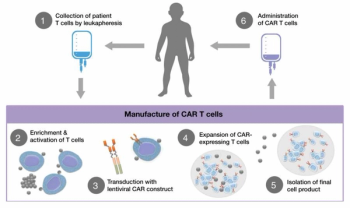
As advanced therapy technology and manufacturing evolve, there are three key operational challenges to address unique to these products.

Despite mounting frustrations in drug R&D for Alzheimer's disease, emerging biopharma firms are still pursuing AD therapies with new science, and different preclinical and clinical trial models.

Exploring new ways to smooth the path toward better participation for patients and other research and healthcare stakeholders.

Regulatory enforcement actions, policy updates, and new guidelines show that ensuring the reliability of clinical data is an ongoing priority.

Click the title above to open the Applied Clinical Trials October 2019 issue in an interactive PDF format.

The transformation in patient perceptions will only happen when the five million Americans who’ve already participated in clinical trials can directly share their experiences with the next five million people considering participating.