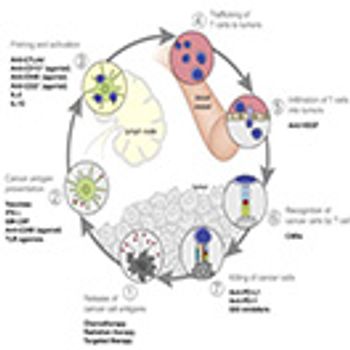
Outlining the three critical challenges that need to be addressed to make personalized cancer treatment a reality.

Outlining the three critical challenges that need to be addressed to make personalized cancer treatment a reality.

Additional policies for registering clinical studies in U.S. and Europe aim to expand access to research data and provide timely information for patients on promising, new treatments.

David Freeman, general manager, Information Ventures, for Quest Diagnostics, talks about the company's use of lab data to create an effective partnership for pharma and biotech companies.

As China’s pharma industry continues to evolve and mature, it is hoped overseas expansion and greater innovation will be exported to serve the interests of patients.

A shift in emphasis in healthcare strategy could reduce attention and funding to therapy and impose tougher controls over research projects, or possible mean a boost for innovative healthcare. A look at where these trends may go.

For drug developers, oncology therapeutics arguably represent both the greatest opportunity and challenge for putting patients at the center. This ebook covers series strategies for Advancing Oncology Drug Development with ePRO Solutions.



Given the wide use of digital technologies in society today, apprehension about implementing eCOA and creating an eCOA back-up plan is unwarranted.




After a court case rehashes the issue of excessive secrecy in trial data, drug firms maintain their objections over releasing data on their products.







The time to stop hammering square pegs into round holes in terms of design strategy has come, as sponsors embrace an adaptive supply chain approach.

With a month left to join one of the 24 ERNs, Peter O'Donnell writes that this initiative is a welcomed demonstration of how cooperation can function in the common interest.
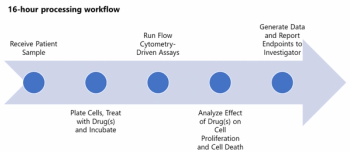
Darren Davis, Senior Vice President at Precision for Medicine, explores the need for specialty labs in cancer treatment plans.





As a new industry survey shows, factoring in a generational cohort surfaces some provocative results related to key health indicators in clinical outsourcing relationships.

