
Peter O'Donnell discusses the European Commission's compendium and how triallists are not only obliged to comply with the current rules, but at the same time to prepare themselves for the future rules.

Peter O'Donnell discusses the European Commission's compendium and how triallists are not only obliged to comply with the current rules, but at the same time to prepare themselves for the future rules.

Click the title above to open the Applied Clinical Trials July/August 2019 issue in an interactive PDF format.

Yaky Yanay, CEO of Pluristem, will discuss his experience and the strategies Pluristem implemented in regenerative medicine clinical trials.

Methods sponsors and CROs can use to minimize the growing pains associated with RBM implementation.

Patient advocates debate whether FDA approval on small, early clinical studies is too fast-tracked for efficacy and safety, or too slow due to long review processes.

Addressing the current hurdles and potential solutions in nonalcoholic steatohepatitis awareness and clinical trial enrollment.

A look at the opportunities and challenges around patient-centric trials and patient centricity in pharma.

The consequences UK faces after being pulled out of the EU without any healthcare and research deal may affect all of Europe.

Navigating the many complexities in clinical trials, manufacturing, and regulatory interaction in moving gene therapies from development to market.
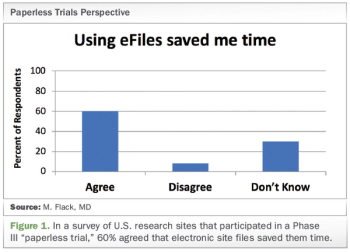
Exploring the use of electronic investigator site files for review of regulatory documents and informed consents.

While obstacles may persist with limited access for patients and researchers, technology can help with this cancer clinical trial paradox.





As pharma businesses shift their focus away from ‘blockbusters’ to treatments for rare diseases, clinical trial design must also go through a period of change and should consider response-adaptive over traditional randomized controlled trial designs.



The possibility of a "no-deal" Brexit scenario would have severe repercussions for clinical trials not only in the UK, but the rest of Europe as well.










