
News


John Ebeid, Senior VP of Randstad Life Sciences writes that though International Clinical Trials Day is a time to celebrate the medical researchers who work to make new discoveries for the good of public health - retaining these employees remains a challenge for the entire industry, especially for today's CROs.


The latest happenings in clinical trial news when it comes to people and business.



Drug development in oncology continues to evolve, Sarah Alwardt, VP of Data, Evidence, and Insight Operations for McKesson, discusses the shift to highly personalized therapies, including new modalities like cell and gene therapies.









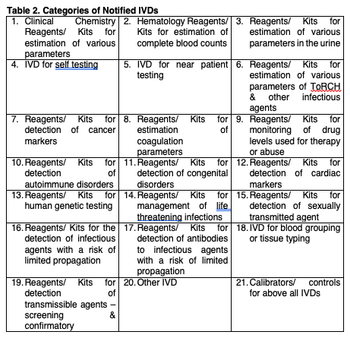
Sudhakar Bangera and Latha MS discuss how in the past medical devices in India were clubbed with regulations for new drugs, biologicals and vaccines, but how new regulations are bringing new and more devices to the area.



The importance of applying change management techniques throughout the preparation, planning, and execution of RBM and RBQM approaches.

Where pharmaceutical interventions for Alzheimer's falls short, nursing care facilities are relying heavily on non-traditional medicines and physical and mental exercise.
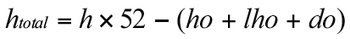
Outlining a resource planning and scheduling model, using the example of an analytical department in drug development.

President and CEO of Cyclacel, Spiro Rombotis, discusses why targeting cyclin-dependent kinases and the DNA repair pathway could enhance care across various oncology indications.

As elections near, politicians in Europe make promises to prioritize the fight against cancer.

FDA leaders urge developers, researchers, and research sponsors to help promote policies and programs to streamline clinical research to develop new medical products at reduced costs.

What small “nudges” can serve as reminders and relevant motivators to help patients meet their goals, talk to a physician, explore a clinical trial, or stick with a treatment?

Click the title above to open the Applied Clinical Trials May 2019 issue in an interactive PDF format.

