
News










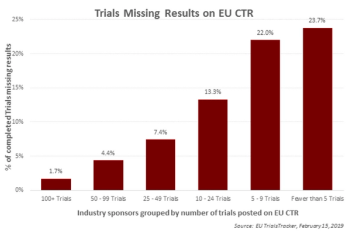
Transparency in clinical trials doesn't have to be difficult but requires attention writes Thomas Wicks, Chief Strategy Officer for TrialScope. Smaller organizations tend to lag in their commitments to clinical data sharing and be non-compliant with regulations, but the trend is shifting.







Dr. Mark Smith, Chief Medical Officer of VistaGen Therapeutics, sits down with Moe Alsumidaie to discuss the challenges of modern psychiatric clinical trial design and implementation and their neuroactive pherine, PH48B.

Cancer therapy development has advanced to researching targeted immunotherapies and moving into gene-specific therapies. Some companies, however, are focusing on reviving cytotoxic therapies that were too toxic for patients when administered generally. Bill Newell, Chief Executive Officer of Sutro Biopharma, sits down with Moe Alsumidaie to discuss the use of a cell-free protein synthesis approach.



Nonadherence in clinical trials plays a significant role in influencing the quality of data, trial results and, subsequently trial cost and duration. It may stem from unintentional drivers, such as forgetfulness, poor organizational skills, protocol regimen complexity, or experiencing an Adverse Event.







