
A look at three contemporary trends that though integrated cautiously at first, may open up a reimagined world of clinical research.

A look at three contemporary trends that though integrated cautiously at first, may open up a reimagined world of clinical research.


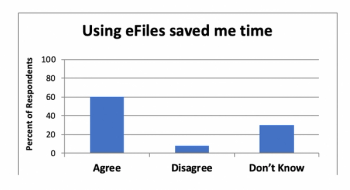
Penelope Manasco, CEO of MANA UBM, answer thorny questions about adopting electronic investigator site files in clinical research.



Bill Tobia, CEO of Clinstruct, Ahmed Bouzid, CEO, Witlingo, and Brielle Nickoloff, Lead Product Marketing, Witlingo, discuss how voice will change the clinical research paradigm.




Penelope Manasco, CEO of MANA UBM, disputes recommendations made by the original authors she finds to be problematic and worthy of further discussion.



David M. Kronfeld, the Head of Real World Data Innovation for Medidata, writes of Mercy Hospital in Joplin, Missouri, that despite seeing over 600 new cancer patients each year, it has historically had very limited access to clinical trials







A look at the latest oncology stats and information most applicable to the clinical trials industry practice.

Researchers and sponsors are looking to use RWE information to help in trial design, product use, developing new therapies, and gaining market approval.

A compilation of recently released news briefs that pertain to the clinical trials industry.

Click the title above to open the Applied Clinical Trials June 2019 issue in an interactive PDF format.

The World Health Organization tries to find a balance with its Road Map for Access on how to best approach research and pricing in Europe.

Undertaking an assessment of the POS can be coupled with the product profile and safety assessments of compounds prior to launching a clinical trial.

Record numbers point to new R&D operating environment, driven by a changing community of sponsors.

Using a risk-based model to navigate the inherent changes and fluctuations in master protocol studies-and help maintain data integrity throughout.

Examining the fundamental changes required to successfully integrate clinical research into mainstream healthcare.