
Regulatory
Latest News

Latest Videos

More News

Ron Lanton, partner, Lanton Law, explains how shifts in policy and government guidance could reduce public participation and complicate the design and recruitment of future vaccine trials.

In this Q&A, Rohit Nambisan, CEO of Lokavant, and Jonathan Crowther, head of predictive analytics, Pfizer, explore how AI is transforming study feasibility, regulatory review, and trial execution.

In this episode of the Applied Clinical Trials Brief, we recap our three most-viewed stories of the previous week with a look into the FDA’s heightened scrutiny of trial design, the wind-down of federal mRNA vaccine programs, and how digital innovation is reshaping the clinical research landscape.

A unified technology approach improves study efficiency and data collection.

In this video interview, Luke Wilson, senior director, biotech, pharma services at Thermo Fisher Scientific, discusses how integrated systems and data transparency enable sponsors to meet evolving regulatory expectations and streamline FDA inspections through enhanced operational readiness.

As clinical trials become more complex, success depends on early collaboration, thoughtful protocol design, and practical strategies to manage risk, reduce delays, and support patients.

In this video interview, Michael Miller, chief operating officer at Quanterix, discusses how biopharma companies—especially smaller biotechs—can leverage platform trials and biomarker-driven accelerated approvals to remain efficient and competitive amid shifting public-private funding dynamics.

In this pilot episode of the Applied Clinical Trials Brief, we examine the renewed push for placebo-controlled vaccine trials in the US, why experts warn it violates core ethical standards, and how these proposals could jeopardize both participant safety and future innovation.

In this video interview, Michael Miller, chief operating officer at Quanterix, outlines key technical and regulatory considerations for clinical operations professionals integrating biomarkers into studies, stressing the importance of clarity on biomarker purpose and working with qualified assay partners.

In this video interview, Kate Gallin Heffernan, life sciences attorney at Epstein Becker Green, advises clinical research organizations to assess their portfolios, diversify funding sources, and strengthen industry-academic partnerships to maintain compliance and continuity amid shifting federal priorities.

Complete Response Letters recently issued by the FDA signal heightened scrutiny of trial design and reinforce the agency’s shifting regulatory expectations for sponsors and CROs.

In this video interview, Kate Gallin Heffernan, life sciences attorney at Epstein Becker Green, advises clinical research teams to closely scrutinize federal fund certifications and document good-faith compliance efforts, even when policies stem from nonbinding executive orders.

In this video interview, Kate Gallin Heffernan, life sciences attorney at Epstein Becker Green, explains how clinical research teams can accurately report on diversity-related grant work while minimizing exposure to False Claims Act scrutiny, by focusing on precise language and framing.

Despite ambitions to streamline regulatory review, FDA’s Elsa platform has been prone to hallucinations, prompting internal scrutiny and questions about AI reliability and governance.

In this video interview, Kate Gallin Heffernan, life sciences attorney at Epstein Becker Green, outlines how even unenforced executive orders can influence federal funding decisions, prompting sponsors and sites to alter public trial messaging to avoid informal scrutiny.

In this video interview, Kate Gallin Heffernan, life sciences attorney at Epstein Becker Green, explains how recent executive orders are creating uncertainty around compliance for clinical research teams receiving federal funding, and why staying alert to agency interpretation is key.
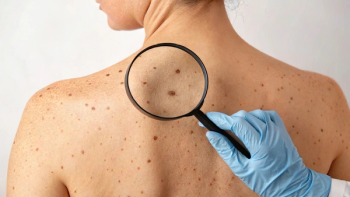
The FDA’s complete response letter cited concerns that the Phase I/II IGNYTE (NCT03767348) trial in advanced melanoma was not an adequate, well-controlled study and that its heterogeneous patient population limited interpretability, preventing approval in its current form.
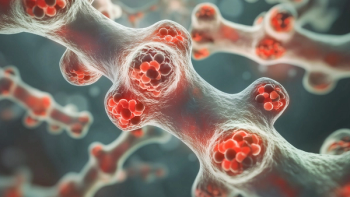
Despite the setback with the Oncologic Drugs Advisory Committee vote, GSK emphasizes the unmet need in multiple myeloma and highlights global momentum behind Blenrep combination approvals.

In this video interview, Ramita Tandon, chief biopharma officer at Walgreens, discusses the operational investments required to activate pharmacies as clinical trial sites, including staff training, regulatory coordination, and patient-facing technology.

Outdated regulations and inflexible sponsor processes are hampering clinical trial recruitment, but empowering sites with modern, compliant marketing tools could turn the tide.

In this video interview, Kyle McAllister, co-founder, CEO, Trially, discusses how clinical trial sites and sponsors are responding to funding constraints by turning to telemedicine, cost-containment strategies, and increased reliance on industry-sponsored research.

In this video interview, Kyle McAllister, co-founder, CEO, Trially, explains how staffing reductions caused by clinical trial budget cuts are threatening patient recruitment and retention and warns of the long-term ripple effects on trial timelines and healthcare innovation.

In this video interview, Kyle McAllister, co-founder, CEO, Trially, discusses how recent federal funding cuts are likely to undermine research focused on underrepresented populations, and why long-term investment in community-based studies is essential to closing persistent health equity gaps.

In this video interview, Judith Ng-Cashin, MD, chief medical officer, Novotech, explores how CRO partnerships are shifting to deliver greater efficiency, cost-effectiveness, and regulatory insight in response to tightening budgets and an increasingly complex clinical research environment.

As clinical research increasingly relies on RWD to enhance trial design and patient insights, tokenization has emerged as a critical solution for securely linking disparate datasets while protecting patient privacy.












