
Lessons learned from COVID-19 vaccine development.

Lessons learned from COVID-19 vaccine development.

Improving receptivity and response to the evolving nature of clinical trial patient oversight.

Implementing new strategies with the use of patient-reported outcomes.

Higher costs headline list of new challenges faced by CROs and sponsors.
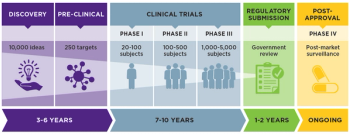
The data, not the plan, provide the direction.
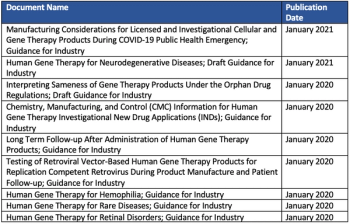
Developing new pathways to overcome study-related challenges is key to realizing the promise of the latest gene therapies.

FDA emphasizes importance of inclusion in trials.

President unveils lengthy program for combatting COVID-19 immediately following inauguration.

Cancer still a primary focus of EMA in light of COVID and vaccine development.

Implementing disclosure and data transparency policies and procedures represents an opportunity for sponsors, rather than an obligation.

Researchers aren't taking full advantage of advances in trial technologies. A lack of clarity in the current version of the International Council for Harmonization "Guidelines for Good Clinical Practice", could be the problem.

Recent webcast addresses what organizations can do to prepare for the next revision of ICH E6.

Looking back on regulatory challenges created by COVID in 2020.

COVID-19 pandemic forces compliancy within organizations following FDA’s 2018 revision of Good Clinical Practice in federal registry.

Battle for trust ensues as vaccines are set for approval.

COVID-19 pandemic shifts traditional process for medicine development in Europe.

FDA’s Vaccines and Related Biological Products Advisory Committee addresses issues related to testing and approval of potential COVID vaccines.

The FDA hints that their increased speed in issuing guidances during the pandemic could become the new norm.

Declarations made by Beate Wieseler don't align with those of many regulators and HTA bodies in Europe and will prove tough to transform.

Though members of the industry have made reassuring comments about progress with candidate COVID vaccines, they acknowledge it could be next year, at best, before any real response would be available.

Though members of the industry have made reassuring comments about progress with candidate COVID vaccines, they acknowledge it could be next year, at best, before any real response would be available.

Fears about overly accelerated development programs has heightened demands for wider access to information on study protocols, statistical analysis plans, and early results.

Study offers clues for installing rapid R&D tactics post-pandemic.
As biopharma companies and research institutes advance the development of promising vaccines against COVID-19, policy makers and health officials have intensified deliberations on strategies for ensuring fair and equitable distribution of anticipated preventives.

FDA to resume “prioritized domestic inspections” for certain regulated products put on hold by COVID-19.