
EMA has launched a "communication perception survey" looking for honest views from among the widest audience on how well it is doing.

EMA has launched a "communication perception survey" looking for honest views from among the widest audience on how well it is doing.

Examining how to optimizing the time and effort of Ethics Committees to efficiently and effectively fulfill their human subjects protection remit.

An amendment to Pediatric Research Equity Act, as part of the 2017 FDA Reauthorization Act, goes into effect soon aiming to change the landscape and promote pediatric cancer drug development.

A look at how real world data and real world evidence are shaping more decentralized trial designs for potential COVID-19 treatments.
As biopharma companies and research institutes advance the development of promising vaccines against COVID-19, policy makers and health officials have intensified deliberations on strategies for ensuring fair and equitable distribution of anticipated preventives.


The need for treatments to combat the spread of COVID-19 is promoting greater cooperation among drug regulatory authorities around the world, with FDA officials communicating more frequently with their counterparts in Europe, Canada, Japan and other nations through established programs and agreements.

Jill Wechsler details the continued rise of incorporating the “patient experience” in drug development measures.

FDA officials make efforts to better manage the increasing amount of applications for new drugs that treat disease in innovative ways and include new kinds of clinical research, starting with a plan to restructure the Office of New Drugs (OND) in the Center for Drug Evaluation and Research (CDER).

Provenance is at the heart of the discussion when it comes to the equation of privacy and provenance.

Jill Wechsler interviews FDA Commissioner Scott Gottlieb about his push for more efficient R&D to help lower drug prices.

The EU General Data Protection Regulation is the most important change in data privacy regulation in 20 years.
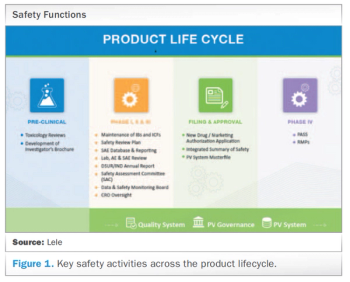
Meeting today’s complex regulatory demands when it comes to drug safety and pharmacovigilance can be especially challenging for small and medium-sized organizations. This report presents the benefits for these companies in outsourcing such activities to functional service providers (FSPs) during clinical trials and post-approval.

In this blog by Peter O'Donnell, the EU provides a report on the progress its made within the pediatric medicines regulation.

The rise of new drugs targeting rare disease ignites increased push for flexible R&D approaches and more collaboration on orphan drug designation and clinical trial design between global regulators.

The language in Europe's Regulation (EU) 536/2014 regarding ethical review of protocols is concerning.

European Parliament has voted through a resolution criticizing the performance of drug companies on pediatric medicines development. This resolution will punish drug companies who neglect to investigate possible pediatric applications of new medicines.

With a new administration in Washington, sponsors and regulators are weighing several initiatives that promise to reshape clinical research policies.

Months of rumblings across Europe that the scheme to promote orphan drugs is being abused by drug firms to maximize profit instead of innovation have been heard. Those rumblings have now turned into shouts.

While approved in 2014, Europe's new regulation governing clinical studies is set to take effect in May-leaving interested parties only six more months to prepare for its arrival.
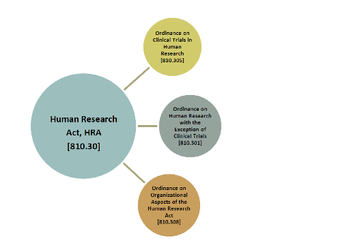
The country’s introduction of a new legal framework has it in sync with European law on clinical research.


Ben Rotz, Director of Medical Transparency at Eli Lilly & Company discusses TransCelerate’s Data Transparency Initiative and position paper regarding patient data de-Identification and anonymization.

The "Cures" bill and PDUFA IV are the latest measures pushing for more systematic inclusion of patient views into drug development.

With patents for many NBCDs soon to expire, the need for regulatory guidance regarding follow-on versions of these products is crucial.