
In part 2 of this video interview, Vincent Keunen, founder & CEO of Andaman7 highlights how technology vendors should be keeping the patient in mind when building solutions.

In part 2 of this video interview, Vincent Keunen, founder & CEO of Andaman7 highlights how technology vendors should be keeping the patient in mind when building solutions.

In a presentation at the 2024 International Association for the Study of Pain World Congress, Concentric Analgesics shared positive Phase II data for its lead candidate.

In part 1 of this video interview, Vincent Keunen, founder & CEO of Andaman7 discusses how service providers and sponsors can make the lives of patients contributing to research easier.

Phase III study of Bayer’s non-steroidal, selective mineralocorticoid receptor antagonist met its primary endpoint.

In the fifth and final part of this video interview, Rama Kondru, PhD, CEO of Veridix AI looks forward to what the LLM landscape in clinical trials could look like in five years.
Phase III study of tirzepatide in adults with heart failure with preserved ejection fraction and obesity met all primary endpoints.

In part 4 of this video interview, Rama Kondru, PhD, CEO of Veridix AI discusses best practices for choosing the right artificial intelligence solutions.

In part 3 of this video interview, Rama Kondru, PhD, CEO of Veridix AI touches on how large language models can leverage unstructured data.
The trial demonstrated clinically meaningful improvements in overall response rate and duration of response in its patient population.

In part 2 of this video interview, Rama Kondru, PhD, CEO of Veridix AI highlights areas that large language models can be used in clinical trials including patient recruitment and retention.

According to the trial data, PGN-EDO51 is outperforming previously studied oligonucleotide therapies.

In part 1 of this video interview, Rama Kondru, PhD, CEO of Veridix AI discusses challenges with adoption and the potential uses of large language models in clinical trials.
AstraZeneca’s CALQUENCE (acalabrutinib) in combination with venetoclax, with or without obinutuzumab achieved the significant and clinically meaningful improvement in the AMPLIFY Phase III trial.

The CETP inhibitor lowered low-density lipoprotein cholesterol by 36.3% at day 84 and 41.5% at day 365 compared to placebo.

Positive trial results show the specialized cell product achieved an overall slowing of disease worsening compared to placebo.

In the fifth and final part of this video interview, Carie Pierce, SVP, global head of growth & business development, DIA touches on how the organization continues to break down silos in clinical research and provide a medium for collaboration.
Pfizer’s giroctocogene fitelparvovec reduced annualized bleeding rate in participants from week 12 up to at least 15 months and achieved superiority compared to prophylaxis.

In part 4 of this video interview, Carie Pierce, SVP, global head of growth & business development, DIA discusses how the organization is looking to expand into different global regions and continue leading awareness for the clinical research industry.

The investigational prophylactic monoclonal antibody met its primary endpoints, reducing infections up to day-150.

In part 3 of this video interview, Carie Pierce, SVP, global head of growth & business development, DIA highlights the most relevant themes related to clinical trials that were present at DIA including DE&I and patient recruitment.

Carie Pierce, SVP, global head of growth & business development, DIA touches on the various themes of the sessions which were held at the 2024 Global Annual Meeting including regulatory, technology, and harmonization.

Dovato regimen met its primary endpoint at 48 weeks for the treatment of HIV-1 in people who are virologically suppressed.

Pierce shares her greatest takeaways from this year's meeting in San Diego, CA.
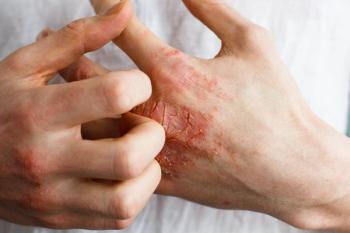
Results of the studies, published in The Lancet, show delgocitinib cream met its primary and all secondary endpoints.
Study met its primary endpoint with statistically significant superior efficacy over 12 weeks of treatment compared to placebo.
The androgen receptor inhibitor significantly increased radiological progression-free survival in combination with androgen deprivation therapy compared to placebo.

Two-year data from duo of Phase III studies show potential for Susvimo as an alternative to eye injections to treat diabetic macular edema and diabetic retinopathy.

Mixed methods study gathered responses from study investigators to identify barriers, solutions, and opportunities associated with continuing critical care randomized trials during the pandemic.

Newly released research report from Information Services Group suggests firms are moving away from traditional, centralized trials in favor of remote technologies.

deLaubenfels addresses key challenges seen by sponsors and highlights the value cough monitoring can provide.