Andy Studna, Senior Editor
Articles by Andy Studna, Senior Editor

Raja Shankar, VP of machine learning at IQVIA, discusses which AI capabilities sponsors are most likely to adopt first to streamline trial workflows and reduce operational burden, while also highlighting emerging applications that could shape the next phase of clinical trial design.

Jeremy Wyatt, CEO, Ametris, discusses how operational best practices for integrating wearables into oncology trials center on minimizing patient burden through thoughtful workflow design, careful device selection, and early planning to ensure digital measures fit the realities of complex patient populations.

Charlie Paterson, partner at PA Consulting, explains how mixed signals on FDA risk tolerance are accelerating the globalization of clinical trial programs and reshaping how sponsors align development activities worldwide.

Industry leaders emphasized that accelerating clinical research must go hand in hand with sustained, community-driven strategies to advance diversity, equity, and inclusion in clinical trials.

Miriam Dervan, founder & CEO of mdgroup, explains how treating patient experience as a strategic investment—rather than a secondary consideration—changes how sponsors, sites, and operational teams design and deliver clinical trials.

Mike Wenger, chief innovation officer at CRIO, discusses how different site types—from academic medical centers to independent research sites—require distinct eSource approaches, and why aligning technology with site workflows is critical to study execution.

Industry leaders explore areas of opportunity for acceleration as well as how early-phase planning, technology, and collaboration can keep moving the clinical ecosystem forward.

Ken Getz of Tufts CSDD and Eliav Barr of Merck discuss how efficiency, public trust, and AI all fit into the current state of clinical research.

Holly Leslie, vice president of services at Ledger Run, explains why site selectivity is not yet universal, but increasingly driven by larger, more sophisticated sites that are demanding stronger remuneration policies and greater leverage in sponsor relationships.

Otis Johnson, PhD, MPA, founder and principal consultant at Vantix Operations, discusses how ESG is evolving from a reporting exercise into an auditable measure of operational readiness, and what that shift means for sponsor expectations, vendor qualification, and supplier governance discipline.

Raja Shankar, VP of machine learning at IQVIA, explains how AI-driven trial simulation and automation are beginning to influence decision-making across every phase of clinical development.

Charlie Paterson, partner at PA Consulting, describes how FDA capacity constraints are creating uncertainty from initial submissions through late-stage approval, elongating timelines and influencing global development strategies.

Charlie Paterson, partner at PA Consulting, outlines how limited FDA guidance on innovative designs, decentralized models, and digital endpoints is forcing clinical operations teams to recalibrate expectations and minimize regulatory risk.
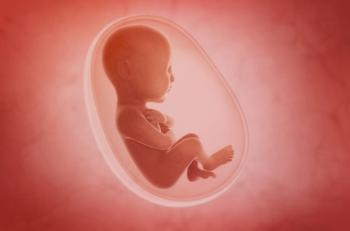
New findings from a Phase II study indicate that antenatal treatment with the FcRn blocker nipocalimab resulted in low fetal drug exposure and transient reductions in infant IgG levels at birth, without evidence of impaired immune recovery or vaccine response through nearly two years of follow-up.

Charlie Paterson, partner at PA Consulting, discusses how fewer new guidance updates are pushing sponsors to rely on historical precedents and non-US standards when making trial design and operational decisions.

In today’s ACT Brief, we examine how reduced FDA capacity is extending regulatory timelines, why sponsors are rethinking operating models to reduce site burden in 2026, and how the FDA and EMA are aligning around guiding principles for artificial intelligence in drug development.

As trials expand into new geographies and decentralized models mature, sponsors are confronting a core operational challenge in 2026: how to scale global execution while reducing system complexity and day-to-day burden on research sites.

The FDA and EMA have aligned on ten guiding principles for the responsible use of artificial intelligence across the drug development lifecycle, establishing a shared framework to support innovation, regulatory consistency, and patient safety.

Charlie Paterson, partner at PA Consulting, explains how reduced FDA capacity and staff turnover have led to longer regulatory timelines, increased preparation for agency interactions, and delayed feedback during early trial planning.

In today’s ACT Brief, we look at how real-world evidence is reshaping trial design rather than replacing trials, what Worldwide Clinical Trials’ acquisition of Catalyst Clinical Research signals for oncology-focused CRO models, and new data showing feasibility and enrollment challenges remain stubborn across global trials.

Worldwide Clinical Trials’ acquisition of Catalyst Clinical Research strengthens oncology expertise and functional service provider capabilities, expanding global trial delivery and flexible resourcing models across early and late phase development.

See how real-world evidence is enabling smaller, smarter, and more efficient trial designs through hybrid models, external comparators, and continuous patient monitoring—while preserving the role of traditional clinical trials.

In today’s ACT Brief, we examine why ESG efforts in clinical development are shifting into vendor oversight, what data and governance barriers still limit broader use of de-identified RWE in submissions, and new Phase II obesity data from Roche as its program moves toward Phase III.

Assess the data quality, linkage, transparency, and auditability challenges that sponsors must overcome to make de-identified real-world evidence fit for regulatory submissions.

In today’s ACT Brief, we explore why decentralized trial innovations struggle to scale without better change management, where real-world evidence most realistically complements traditional trials, and how efficiency, AI, and platformization are expected to reshape clinical operations in 2026.

A look at how efficiency, access, platformization, AI, non-traditional players, and regulatory recovery are expected to reshape clinical operations in 2026.

Understand where real-world evidence most effectively complements or substitutes traditional trial data, from post-market surveillance and label expansion to challenging areas such as rare disease research.

In today’s ACT Brief, we examine how large de-identified datasets are reshaping trial design and site strategy, why global pharmaceutical production is expected to cool after a tariff-driven surge, and what FDA’s new M4Q(R2) draft guidance means for quality submissions.

Explore how large-scale, de-identified real-world datasets enable more representative trial design, improve site selection, and support patient identification beyond the limits of traditional clinical study populations.

In today’s ACT Brief, we look at how FDA policy is accelerating the use of de-identified real-world evidence in clinical development, why a new bipartisan funding package could stabilize federal research agencies, and how the US withdrawal from the World Health Organization reshapes global health coordination.






























