
Some can't miss sessions for the upcoming meeting.

Some can't miss sessions for the upcoming meeting.

Study Connect™ App Enables the Mobile Management of Randomization, Inventory Management and Unblinding in Clinical Trials

ERT, a provider of high-quality patient safety and efficacy endpoint data collection, cloud analytics and workflow solutions, announced the availability of a new solution to wirelessly integrate ECG data collection with an electronic Clinical Outcome Assessments (eCOA) tablet.

Certara®, a global biosimulation technology-enabled drug development consultancy, announced that it is endowing a new three-year, full-time position – the Certara Lecturer in Precision Dosing – at the Manchester Pharmacy School, The University of Manchester, England.

IAOCR, a provider of independently recognized, competence-based accreditation, consultancy and accredited training for clinical research professionals, offered comment the new EU regulation for clinical trials planned for implementation May 28, 2016.

eClinical technology services provider Clinerion and the Alliance for Clinical Research Excellence and Safety (ACRES) announced their partnership for accelerating patient recruitment and promoting risk-based quality management.

The DIA has an app to help navigate meetings from your smart device, and has been updated this year.

TrialScope, a provider of clinical trial transparency and compliance solutions, announced a clinical trial sponsor has selected its PharmaCM clinical trial disclosure platform.

Run or walk with CISCRP to raise awareness of volunteers who give the gift of their participation in clinical research and make new medical discoveries possible.

PAREXEL announced enhancements to its Randomization and Trial Supply Management (RTSM) service.

With the DIA housing program, there is such a thing as a free breakfast at select DIA Hotels.

Eisai announced that, in order to further promote transparency in clinical trial data disclosure, it has determined its policy on clinical trial data disclosure and is making clinical trial data publicly available to researchers via an external website.

BioClinica®, Inc., a specialty clinical trials services and technology provider, has partnered with advisory and outsourcing services company Kinapse on BioClinica’s Compass Intelligent Monitoring solution.

inVentiv Health, a global provider of clinical development and comprehensive commercialization services, announced expansion of compliance products and services to assist biopharmaceutical companies conducting non-interventional studies globally.

eCOA provider CRF Health is currently providing two special DIA conference discount offers.

Quotient Clinical has announced results from an Enabled-First-in-Human (Enabled-FIH) program conducted for the Janssen WAVE Early Development unit.

Make sure your perspective is heard, and get valuable insights and data for your TMF improvement initiatives

Theorem Clinical Research announced it will open a new, state-of-the-art clinical supplies facility in Frankfurt, Germany, in June.
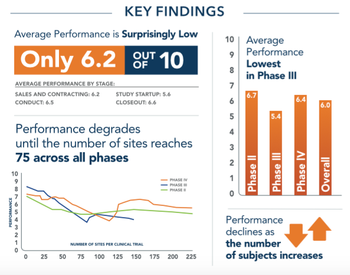
Graphics and data regarding the current state of the Clinical Trials industry.

Radiant Sage, a provider of on-demand clinical trial imaging infrastructure solutions, announced its Event Adjudication Committee (EAC) solution for its RadClinica Clinical Trial Management System (CTMS).

CRO Analytics, a provider of validated clinical trial performance data, announced a partnership with the Association of Clinical Research Professionals focused on measuring investigator site personnel views of clinical trial quality


The Society for Clinical Research Sites (SCRS) announced a two-year collaboration with Myoderm, a sourcing, distribution, and management of comparator drugs and other pharmaceutical products and supplies for clinical research organization.

Research from MediciGlobal shows that patients who actively sought clinical trial involvement through its online recruitment model had a 38% lower relative risk of drop out across four studies compared to those who were recruited by sites, with divergence across visits.

Medidata announced the completion of an open-source connector linking Apple® ResearchKitTM with its Medidata Clinical Cloud® platform.

The ACRES' Accountable Research Blog is being piloted in the San Antonio, Texas market, and is projected to be extended to other Hearst media markets, both domestically and internationally.

The SCRS Site Scholarship Program awards membership to qualifying sites striving for excellence in clinical research who are not current or previous SCRS members.

Providing Customized Full-Service Clinical Trial Operations

PHT announced it has selected the Hewlett Packard (HP) ElitePad 1000 G2 with Windows 8.1 for its SitePad® System.

ICON has announced Firecrest eConsent, an electronic informed consent solution that incorporates recommendations from the FDA¹s recent draft guidance on informed consent.