
Quorum Review IRB is introducing a comprehensive program that supports the needs of Phase I Healthy patients.

Quorum Review IRB is introducing a comprehensive program that supports the needs of Phase I Healthy patients.

With the Perceptive MyTrials Analytics solution, clinical trial sponsors can now use a mobile-enabled, single entry-point to access predictive data analytics.

To mark the 50th anniversary of the adoption of the first European Union (EU) pharmaceutical legislation, the European Commission is organizing a one-day conference that will take place in Brussels September 28.

Wingspan Technology now offers an eTMF Readiness Assessment program to help sponsors and CROs assess their level of readiness for implementing eTMF.

According to the release, PPD noted that wearable technology will have the potential to benefit Novartis and its other clients because of the integration with the technologies patients use on a daily basis.

The program is designed to facilitate dialogue between industry stakeholders and clinical research sites.

Designed to ensure patients and patients in clinical trials safely receive their proper medications, the regulation serves as a verification measure to enforce the European Union’s Falsified Medicine Directive.

The new Clinical Conductor contains a myriad of enhancements that foster more precise reporting and analysis.

An independent judging panel will select three finalists from the submissions.

OmniComm Systems, a global provider of clinical data management technology and services, announced that it has signed a reselling partnership agreement with Tri-I Biotech Shanghai Inc.

Almac Group announced the successful completion of the Health Sciences Authority (HSA) inspection of its new Asia Pacific headquarters in Singapore.

Parexel announced expanded services and capabilities in model-based drug development (MBDD) through its Quantitative Clinical Development (QCD) group.

Certara®, a global biosimulation technology-enabled drug development company, announced the merger of its consulting group, Pharsight Consulting Services.

In response to increasing demand of Sharp Clinical Services.

goBalto, Inc., a provider of cloud-based clinical study startup solutions, announced its latest version of goBalto Activate.

Quintiles and Quest Diagnostics announced the launch of Q2 Solutions, their new combined clinical trials laboratory services organization.

The Rethink Research Competition has opened voting for its People’s Choice Award.

The pilot involved several CDER employees working together with OEA employees to respond to drug related inquiries posted on FDA’s Facebook page.

BioClinica announced the opening of new and expanded offices in London and Munich.

A team of scientists at the University of Vermont has invented a new technique for discovering potentially dangerous drug interactions and unknown side-effects using Twitter hashtags.
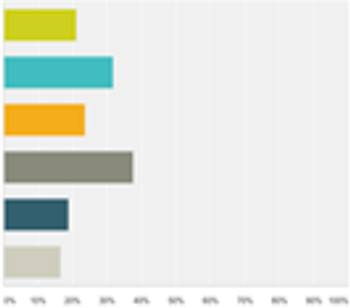
A recent survey conducted by Applied Clinical Trials and SCORR Marketing on mHealth in Clinical Trials found the majority of respondents believe mHealth is only beginning its impact on our industry. To the question of challenges to implementing a mHealth strategy, clinical research professionals responded that having the knowledge to execute the skills was the leading challenge.

Acorn Regulatory, an ISO-certified medical device and pharmaceutical consulting firm, has customized programs to help U.S. manufacturers of drug-device combinations in meeting European regulatory approvals.

Medidata announced that its cloud-based technology platform has been adopted by JCR Pharmaceuticals Co., Ltd.

WIRB-Copernicus Group® (WCG), a provider of regulatory and ethical review services and software to support clinical research, announced its 13th annual WCG International Fellows Program in Research Ethics now includes ethics training at New York University (NYU) Langone Medical Center.

Bracket Global, and Clintara announced the two companies have agreed to combine.

The Society for Clinical Research Sites (SCRS) announced a two-year partnership with LabCorp.

The Tufts Center for the Study of Drug Development (CSDD) released a new report highlighting innovative approaches across the R&D continuum that pharmaceutical companies are implementing to drive efficiency and lower cost.

Oracle Health Sciences announced the availability of Oracle Health Sciences InForm Cloud Service 6.1.

Catalent Singapore facility is awarded GMP certification.

Medidata announced the completion of MOVE-2014, a behavioral study it sponsored to test whether mobile health (mHealth) devices and tools could be used to drive better health outcomes in overweight adults with Type-2 Diabetes.