Updated GLISTEN trial results presented at EASL 2025 confirm that the IBAT inhibitor linerixibat significantly improved worst itch scores and itch-related sleep interference over 24 weeks in adults with primary biliary cholangitis.

Updated GLISTEN trial results presented at EASL 2025 confirm that the IBAT inhibitor linerixibat significantly improved worst itch scores and itch-related sleep interference over 24 weeks in adults with primary biliary cholangitis.

Updated results from the Phase III ELATIVE trial reveal Iqirvo (elafibranor) significantly reduced fatigue in patients with primary biliary cholangitis, with effects independent of pruritus and supported by proteomic data.
Topline results from the Phase III DESTINY-Breast11 trial show that Enhertu (trastuzumab deruxtecan) followed by paclitaxel, trastuzumab, and pertuzumab significantly improves pathologic complete response rates compared to anthracycline-based standard-of-care regimens in the neoadjuvant treatment of high-risk, locally advanced HER2-positive early-stage breast cancer.

A Phase III randomized trial published in JAMA Network Open found no significant difference in symptom improvement between onabotulinumtoxinA injections and midurethral sling surgery in women with moderate to severe mixed urinary incontinence, with many ultimately needing both therapies to sustain relief.
New long-term extension data from the Phase IIb/III QUASAR trial demonstrate that Tremfya (guselkumab) maintains durable clinical and endoscopic remission through 92 weeks in adults with moderately to severely active ulcerative colitis, with consistent efficacy across prior biologic and JAK inhibitor exposure.
Interim results from the Phase III ESSENCE trial published in The New England Journal of Medicine show once-weekly semaglutide improved liver histology, metabolic markers, and weight loss in patients with biopsy-confirmed metabolic dysfunction–associated steatohepatitis and stage 2 or 3 fibrosis.
Phase III trial data published in The New England Journal of Medicine support Nucala’s role as an add-on biologic therapy to lower exacerbation rates and delay disease progression in patients with eosinophilic chronic obstructive pulmonary disease receiving standard inhaled treatment.
Rinvoq (upadacitinib) becomes the first oral JAK inhibitor approved by the FDA for the treatment of giant cell arteritis in adults, following robust data from the Phase III SELECT-GCA trial demonstrating its efficacy in achieving sustained remission and reducing glucocorticoid exposure.
Early findings from Johnson & Johnson’s Phase IIb SunRISe-1 trial show the promise of TAR-200 in patients with Bacillus Calmette-Guérin–unresponsive, high-risk non–muscle-invasive bladder cancer, highlighting its potential as a non-surgical treatment alternative.
Interim KEYNOTE-689 trial data show that perioperative Keytruda significantly lowers the risk of disease progression or recurrence in resectable, locally advanced head and neck squamous cell carcinoma, marking the first major clinical advance for this patient population in more than two decades.
Lorundrostat, a selective aldosterone synthase inhibitor, significantly reduced systolic blood pressure in patients with uncontrolled and treatment-resistant hypertension in the Phase II Advance-HTN trial, indicating its potential as a safer, more effective alternative to current therapies.
Brensocatib significantly reduced the rate of pulmonary exacerbations and slowed lung function decline in patients with non-cystic fibrosis bronchiectasis in the Phase III ASPEN trial.

In the Phase III SELECT-GCA trial, Rinvoq (upadacitinib) 15 mg with a 26-week glucocorticoid taper significantly improved sustained remission rates and reduced disease flares in patients with giant-cell arteritis, offering a promising glucocorticoid-sparing treatment option with a favorable safety profile.
Tolebrutinib did not show superiority over Aubagio (teriflunomide) in reducing relapse rates or MRI-detected inflammatory activity in relapsing multiple sclerosis, but early signals suggest it may have potential in slowing disability progression, warranting further long-term and comparative studies.
In the Phase III DESTINY-Breast09 trial, Enhertu (trastuzumab deruxtecan) and Perjeta (pertuzumab) significantly improved progression-free survival compared to the current standard of care in the first-line treatment of HER2-positive metastatic breast cancer, suggesting a potential new standard of care.
In Phase III MINT trial, Uplizna (inebilizumab) demonstrated significant short-term efficacy and a manageable safety profile in adult patients with generalized myasthenia gravis, offering a promising B-cell–targeting treatment option pending long-term data.
Beqvez (fidanacogene elaparvovec), an FDA-approved one-time gene therapy for hemophilia B, demonstrated sustained factor IX expression, low bleeding rates, and a favorable safety profile over long-term follow-up.

Adding Farxiga (dapagliflozin) to standard care significantly reduced the risk of death or worsening heart failure in older patients with severe aortic stenosis following transcatheter aortic-valve implantation, with consistent benefits across patient subgroups and a favorable safety profile.
Tolebrutinib, a brain-penetrant BTK inhibitor, may fill a significant unmet need by delaying disability progression in patients with non-relapsing secondary progressive multiple sclerosis.
In the Phase III EXPECTS trial, patients experiencing mostly mild posterior circulation ischemic stroke who were ineligible for thrombectomy showed significantly better functional recovery at 90 days when treated with alteplase between 4.5 and 24 hours after stroke onset, without an increased risk of symptomatic intracranial hemorrhage.
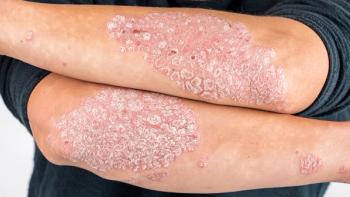
Long-term extension data show Tremfya (guselkumab) significantly reduced symptoms and inhibited structural joint damage progression in patients with active psoriatic arthritis.
Phase III SOUL trial shows daily oral semaglutide lowers the risk of major adverse cardiovascular events in patients with type 2 diabetes and atherosclerotic cardiovascular disease or chronic kidney disease, with benefits consistent with injectable semaglutide and other GLP-1 receptor agonists.
The Phase III API-CAT trial found that extended anticoagulant therapy with reduced-dose Eliquis (apixaban) was noninferior to the full-dose regimen in preventing recurrent venous thromboembolism in patients with cancer.
Oral PCSK9 inhibitor, AZD0780, demonstrated a significant 50.7% reduction in LDL cholesterol levels in the Phase IIb PURSUIT trial when added to standard statin therapy.
Eli Lilly’s lepodisiran, an investigational siRNA therapy, achieved significant and durable reductions in lipoprotein(a) levels, a major genetic risk factor for cardiovascular disease.
The Phase III MITIGATE trial demonstrated that Uplizna (inebilizumab) significantly reduces the risk of flares, lowers glucocorticoid dependence, and increases rates of treatment- and glucocorticoid-free remission in patients with immunoglobulin G4-related disease.

Solriamfetol achieved the primary and key secondary endpoint of the Phase III FOCUS trial by significantly lowering attention-deficit hyperactivity disorder symptoms and disease severity in adults compared to placebo, with a favorable safety and tolerability profile.
FDA grants Priority Review status to Sanofi's tolebrutinib for the treatment of non-relapsing secondary progressive multiple sclerosis based on positive findings from multiple Phase III trials.
Phase III VERITAC-2 trial results show vepdegestrant significantly improved progression-free survivalcompared to fulvestrant in patients with ESR1-mutant (ESR1m) advanced or metastatic breast cancer, but did not achieve statistical significance in the overall intent-to-treat population.
Phase III IMvoke010 trial found that maintenance treatment with Tecentriq (atezolizumab) did not significantly improve survival compared with placebo in patients with locally advanced squamous cell carcinoma of the head and neck following multimodal definitive treatment.