Cell-selective, adeno-associated virus gene therapy DB-OTO restored hearing to normal levels within 24 weeks in a child born with profound genetic deafness due to variants of the otoferlin gene.

Cell-selective, adeno-associated virus gene therapy DB-OTO restored hearing to normal levels within 24 weeks in a child born with profound genetic deafness due to variants of the otoferlin gene.
Phase I/IIa HORNBILL trial results show Boehringer Ingelheim’s novel humanized monoclonal anti-Sema3A antibody was well tolerated and showed early signs of efficacy.
Johnson & Johnson’s novel targeted therapy TAR-200 produced a greater than 80% complete response rate in patients with Bacillus Calmette-Guérin–unresponsive, high-risk non–muscle-invasive bladder cancer with carcinoma in situ.
The combination of Calquence (acalabrutinib; AstraZeneca) plus bendamustine and rituximab generated a clinically meaningful improvement in progression-free survival in the first-line treatment of adults with mantle cell lymphoma.
Phase III KEYNOTE-811 trial data show Keytruda (pembrolizumab) combined with trastuzumab and chemotherapy achieved the primary endpoint of overall survival in the first-line treatment of patients with HER2-positive locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma.
Data from the Phase III STRIDE-10 trial showed V116 produced immune responses against the serotypes responsible for the majority of adult invasive pneumococcal disease.
Enhertu, a HER2-directed antibody-drug conjugate, showed a clinically meaningful survival benefit in progression-free survival among patients with HR-positive, HER2-low metastatic breast cancer.
Phase II/III COMPANION-002 trial shows promise of CTX-009 in combination with paclitaxel for patients with metastatic or locally advanced biliary tract cancer.

Rinvoq (upadacitinib) shows efficacy in both itch resolution and skin clearance in the treatment of patients with atopic dermatitis.
Data from part one of the Phase III RUBY trial show Jemperli (dostarlimab) plus chemotherapy produced a statistically significant and clinically meaningful survival benefit in patients with primary advanced or recurrent endometrial cancer, including those with mismatch repair proficient/microsatellite stable tumors.
Data from the Phase Ib portion of the KOMET-001 trial showed that the once-daily oral treatment may provide a substantial improvement over available therapies for relapsed/refractory NPM1-mutant acute myeloid leukemia.
Morbidity and Mortality Weekly Report finds that the original monovalent COVID-19 vaccines were associated with fewer hospitalizations, particularly within the first four months after vaccination, but the duration of protection from the original vaccine diminished over time.
Meta-analysis shows neoadjuvant immune checkpoint inhibitors with chemotherapy improves two-year event-free survival and pathologic complete response in patients with early-stage non–small cell lung cancer.
Data from an open-label extension study show that administration of first-line Kesimpta for up to six years in treatment-naïve patients recently diagnosed with relapsing multiple sclerosis led to fewer relapses, suppressed MRI lesion activity, and fewer disability worsening events.

Phase III SURMOUNT-OSA trial data shows promise of tirzepatide, marketed as Zepbound and Mounjaro, treating the underlying disease in patients with moderate-to-severe obstructive sleep apnea.
Imfinzi (durvalumab; AstraZeneca) plus gemcitabine and cisplatin was found to double the overall survival rate after three years in patients with advanced biliary tract cancer.
Columvi with gemcitabine and oxaliplatin showed the potential to improve survival in patients with relapsed or refractory diffuse large B-cell lymphoma who are not candidates for autologous stem cell transplant.

Multicenter, open-label, 156-week extension trial finds that Qulipta (atogepant) reduced migraine days and acute medication use in patients with chronic or episodic migraine.
A full summary of the acute adverse effects of psilocybin in the treatment of depression and anxiety is vital for health care providers to provide effective patient counseling.
Findings from the Phase I/II KRYSTAL-1 trial of Krazati (adagrasib) plus cetuximab showed promising clinical activity and tolerable safety in patients with previously treated KRASG12C-mutated locally advanced or metastatic colorectal cancer.
Findings from MONeT clinical trial show positive safety and efficacy of Abrysvo in individuals aged 18 to 59 years with an increased risk of developing severe respiratory syncytial virus-associated lower respiratory tract disease.
In the EMPACT-MI trial, Jardiance produced a 10% relative risk reduction in the primary composite endpoint of time to first hospitalization for heart failure or all-cause mortality.
Trial to analyze novel therapy MK-1084 plus Keytruda (pembrolizumab) in certain patients with metastatic non-small cell lung cancer whose tumors harbor KRAS G12C mutations and express PD-L1.
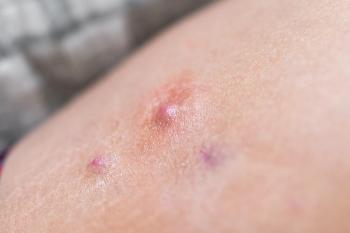
In the Phase III BE HEARD I and BE HEARD II trials, a greater proportion of patients with moderate-to-severe hidradenitis suppurativa who were administered Bimzelx achieved the primary endpoint of clinically meaningful improvements in HiSCR50.
Raludotatug deruxtecan is a potential first-in-class CDH6-directed antibody drug conjugate that has shown promise in patients with CDH6-expressing serous-type ovarian cancer and renal cell carcinoma.
The investigational TROP2-directed antibody drug conjugate improved progression-free survival in patients with unresectable or metastatic HR-positive, HER2-negative breast cancer.
Phase III GATHER2 trial data show Izervay reduced the rate of geographic atrophy lesion growth across every month and every-other-month dosing through two years of treatment.
Krazati (adagrasib) was previously granted accelerated approval by the FDA to treat patients with KRASG12C-mutated locally advanced or metastatic non-small cell lung cancer who were previously administered at least one systemic therapy.

LPCN 1148 is an oral treatment that has shown promise preventing recurrence of overt hepatic encephalopathy and treating sarcopenia.

Johnson & Johnson is moving forward with a pair of Phase III trials of nipocalimab to reduce the risk of fetal neonatal alloimmune thrombocytopenia in alloimmunized pregnant patients.