
Global clinical trial landscape changes pose new challenges for all sites-even AMCs.
Executive Director and Professor, Tufts University School of Medicine

Global clinical trial landscape changes pose new challenges for all sites-even AMCs.

A look at the vague but time consuming requirements imposed on sites and efforts to ease the burden.

Pilot test to rebrand clinical research shows promise as a way to build public trust and promote interest.

Individual countries hold the key to finding hot spots in growth regions like Central and Eastern Europe.

Lack of adequate and attentive support is preventing some sites from fully taking advantage of EDC and ePRO technologies.

A growing trend in the industry has many pharmaceutical companies looking to manage the changing mix of global clinical trial locations.

The FDA's Critical Path Initiative acknowledges long-standing and widely accepted challenges facing the clinical research enterprise and identifies several opportunity areas that may help to address these challenges.
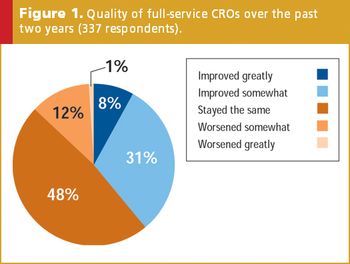
Following more than two decades of growing involvement in nearly all aspects of drug development, full-service and niche-services contract research organizations (CROs) have become a common and integral part of most sponsor companies' clinical research teams.
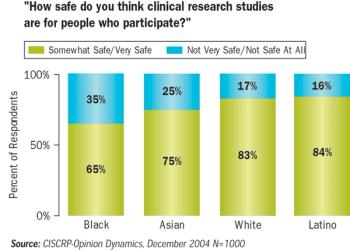
During the past decade, spending on patient recruitment programs has grown 16% annually. In that same period, volunteer randomization rates have declined.