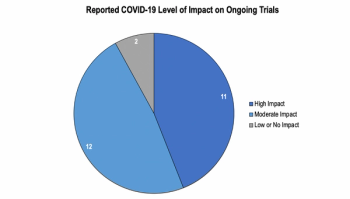
Findings from a Tufts study examining the effects of COVID-19 on clinical trials.
Executive Director and Professor, Tufts University School of Medicine

Findings from a Tufts study examining the effects of COVID-19 on clinical trials.

Study offers clues for installing rapid R&D tactics post-pandemic.

AI presents rare opportunity to assess the scope of this longtime challenge.
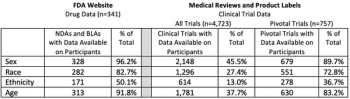
Diversity in clinical trials is an important part of developing new medications that are safe and effective for all potential patients, understanding the demographic disparities within them will provide several benefits to the field.

Study holds new benchmarks and improvement opportunities.

Higher screen failure and patient dropout rates are raising the imperative to pilot and implement new study conduct models in trial recruitment and retention.

Interest in benchmark data on the scope, performance, and economics of rare disease drug development efforts has grown.

Global experts collaborate to form the Addressing Lupus Pillars for Health Advancement (ALPHA) Project in order to combat challenges in lupus drug development.

New study reveals that inconsistent, tactical, and reactive outsourcing practices predominate.

Amid industry feedback that the growing volume and diversity of eClinical data collected for studies is taxing cycle times, two studies highlight the need to optimize protocol design and executional complexity to overcome these data management burdens.

Study examines the growing integration of real-world data and evidence and the remaining roadblocks to adoption.

Recent study, and others in literature, inform misconceptions around physician and nurse involvement in clinical trials.

Traditional and new players are seeing growth and opportunity as the integration of clinical research and healthcare intensifies.

The nascent and fragmented global community of investigators is showing signs of scaling and maturing.

As Applied Clinical Trials celebrates its 25th birthday, longtime contributing author and columnist Ken Getz looks at the four truisms that still drive clinical trial operations today and into the future.

Examining the evolution of protocol design and collaboration executing strategies.

Recent studies may help break down barriers to implementing patient-centric initiatives.

New study finds that the long-strained relationship between sites and institutional review boards may finally be improving.
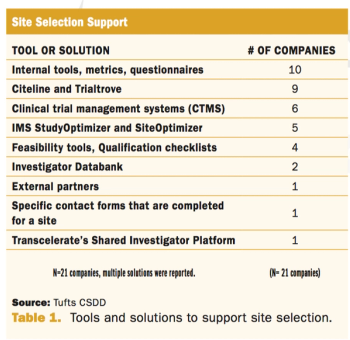
Assessing practices and inefficiencies with site selection, study start-up, and site activation.

New study finds that most biopharma companies are now using a standard set of key performance indicators.

Study collects first comprehensive metrics on current supply management and distribution practices.
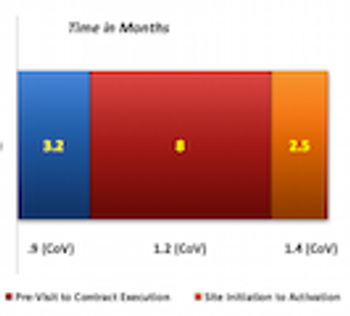
Study finds that despite the use of new approaches to streamline and accelerate study start and improve site selection and activation, the impact on start-up cycle times has been limited. The reasons why are explored.

Research reveals a need for sponsors to be much more consistent, disciplined and focused in their CRO usage.

Work burden and performance hurt by technology incompatibility.

Study provides real metrics on the trial-scope affects of protocol changes-shedding fresh light on the importance of adopting new strategies to reduce select amendments.

Despite wealth of data collected, many inefficiencies exist in the site-sponsor transfer of insights from clinical trials.

Study uncovers subtle distinctions in attitudes and perceptions among the two groups.

Survey provides new benchmarks on e-solutions adoption, but actual impact data remains elusive.
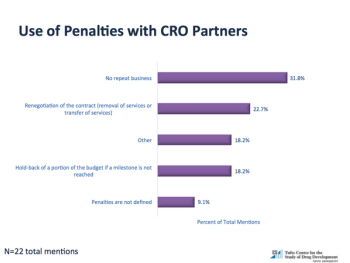
Demand for outsourced services to provide clinical development capacity and expertise has grown substantially over the last 10 years.

While still early days, key metrics can be collected in three broad areas