
Study takes rare look at the financial and resource burden for sites in managing regulatory compliance.
Executive Director and Professor, Tufts University School of Medicine

Study takes rare look at the financial and resource burden for sites in managing regulatory compliance.

Study shows that industry contributions to R&D go well beyond the applied area of clinical testing.

Sponsor companies face intense pressure to deliver higher levels of efficiency and drug development performance. A growing number of sponsors are now acting on the belief that improvements in protocol design feasibility hold the key to addressing and easing some of these pressures.


Research shows that the potential of integrated alliances remains elusive in the near term.

Pressure to shorten study start-up timelines puts clinical supply management in the crosshairs.

Unique feedback from patient survey could help inform future clinical supply design and implementation.

Jean Burns shared experiences with me that offer important insights for government and industry funded clinical research sponsors to consider as they look to improve their partnership with study volunteers.

Adaptive trial designs have the potential to transform success rates, but require new operating strategies and practices.

Public's view of clinical research has improved during the past five years, CISCRP survey reveals.

Active evaluation of non-core procedures is an unusual winwin opportunity for sponsors and CROs.

As integrated alliances assume more risk, novel strategies and practice must follow.
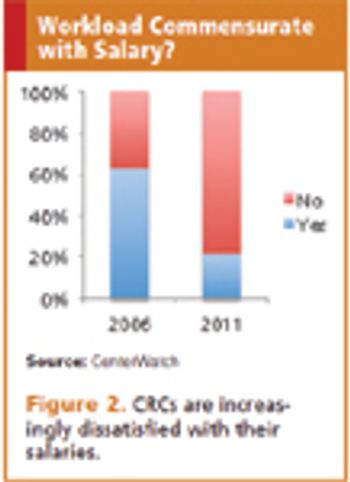
Study coordinators see responsibilities increase dramatically while salary levels remain flat.

From tactical to strategic: tracking the evolution of global clinical supply chain management.

Despite their growing role, little benchmark management data, until now, existed on global study monitors.
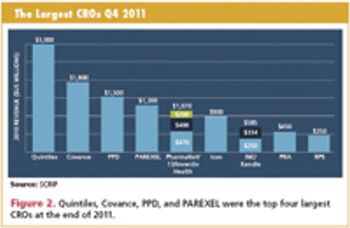
Continual change is coming as sponsors' sourcing requirements diverge from the major CROs.

Better selection and tougher site management is not enough to improve enrollment success.
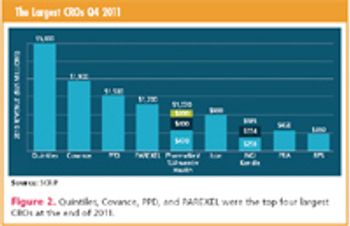
Continual change is coming as sponsors' sourcing requirements diverge from the major CROs.

Compliance with reporting clinical trial results is low, and an even larger opportunity is being missed.

Research shows four factors best predict successful patient enrollment in clinical trials.

Collaborative communities may hold the key to transforming a half-century's old R&D paradigm.

Direction from regulatory agencies would help eradicate wasteful 100 percent source data verification.

Doing more harm than good will ultimately force human subject protection system reform.

Why investigative sites are at financial risk and how it may effect sponsors and CROs.

A recent survey indicates pharmacists should provide more clinical trial information to patients.
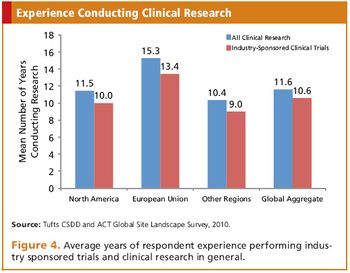
New survey from Tufts CSDD and Applied Clinical Trials provides an inside look at global sites.

Reductions in unused data will improve study performance, lower costs, and address ethical concerns.

Mergers and acquisitions volume is way up, but have they fulfilled their intended purpose?
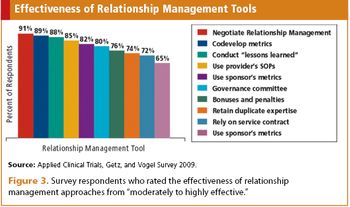
New survey captures sponsors changing global usage of, and relationships with, CRO partners.

Out of necessity, providers may finally become valued strategic partners.