
Valerie Powell, Vice President of Research Services at HealthiVibe, discusses her experience with obtaining patient insights and helping her clients apply them to trial design and conduct in a pandemic.

Valerie Powell, Vice President of Research Services at HealthiVibe, discusses her experience with obtaining patient insights and helping her clients apply them to trial design and conduct in a pandemic.

FDA is reviewing the record of its accelerated approval program following recent withdrawals of certain key indications for several leading cancer therapies, based on the failure of post-approval studies to document extended benefits of treatment.

A new partnership, to be known as EP PerMed, aims to bring public and private sector interests together.

Federal Trade Commission to launch a broad review of drug-company mergers.

Dr. Peter St. Wecker, Director of Clinical Process Improvement & Innovation at Zogenix, offers his unique perspective on challenges and changes that sponsors and sites underwent during the COVID-19 pandemic.

Concerns mount among regulated industry stakeholders as White House stalls on naming FDA permanent commissioner.

With the cancellation of most field site visits last year due to the coronovirus pandemic, FDA now faces a serous backlog in inspections both in the US and abroad.
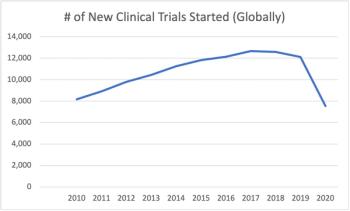
Though the industry was expected to boom with new studies—data shows a significant drop in the amount of new trials started in 2020

Upcoming study reveals challenges faced by Europe over the pricing of pharmaceuticals.

Pamela D. Garzone, Chief Medical Officer at Calibr, shares her perspectives on how investor decisions are shifting dramatically into biopharmaceuticals.

Vaccine manufacturers anticipate significant boosts in output in the coming weeks.

The latest initiative against COVID, the European Health Emergency Preparedness and Response Authority, is taking shape with intentions of revealing the vulnerabilities in global supply chains and more.

FDA issues a sharp warning letter rebuking AcelRx Pharmaceuticals for its ”glib and simplistic” messaging on painkiller Dsuvia.

After three years, member states of the EU have yet to make any real progress on legislation that would coordinate their clinical assessments more closely.

Jane Myles, Director of Decentralized Trials at Covance by LabCorp, and former Head, Operational Intelligence and Innovation at Roche, discusses her journey as a clinical trial participant.

Despite challenges and disruptions posed by the coronavirus pandemic this past year, FDA annual reports on drug regulatory programs and policies confirm successful efforts for meeting review time frames and updating policies and programs.

Jessica Lee, SVP Clinical Operations and Global Integration at Inovio Pharmaceuticals discusses her perspective on the emergence of novel COVID variants and how they could impact results for sponsors currently running COVID-19 studies

R&D on medical treatments lags notable success with vaccines.

More access to trials, an increase in digitalization, and much more are on the horizon for the industry in 2021.

The selection of a new FDA commissioner has become a contentious issue in Washington. Jill Wechsler reports.

John Reites, CEO of THREAD Research, discusses how data collection is changing as the industry moves towards decentralized approaches.

The $4 billion program will embrace the disease's entire pathway, from prevention to quality of life of patients and survivors.

Amy Davis, Sr. Director of Clinical Development Oncology at Eli Lilly, discusses her perspectives on diversity in clinical trials and the workplace.

With Democrats controlling Congress and the White House, expectations are high that policy makers will revise certain coverage and payment policies. Jill Wechsler reports.

The process of distributing millions of doses of new vaccines across the nation has been much less effective than anticipated.

After issuing a conditional marketing authorization issued just a week ago for the Moderna vaccine and receiving applications for more, the EU has reserved vaccines sufficient to treat more than 80% of the European population.

The first step is admitting there's a problem, now what will the industry do about it?

Applications put on hold as agency limits alternative oversight methods.

CDER's process for vetting and authorizing important new therapies remained strong and productive last year, despite the need to deal with COVID-19 related approvals and policies.

As the EMA gives Moderna's COVID vaccine the thumbs up, the European Commission has quickly followed up with its own approval.