
While the clinical trials industry has always been a slacker in innovation, we'll soon start seeing automation and artificial intelligence significantly impacting the way biopharmaceutical enterprises run clinical trials, says Moe Alsumidaie.

While the clinical trials industry has always been a slacker in innovation, we'll soon start seeing automation and artificial intelligence significantly impacting the way biopharmaceutical enterprises run clinical trials, says Moe Alsumidaie.

Therapies aimed at correcting genes that cause diseases are gaining ground in biopharma, some are even specializing in specific mechanisms of action by targeting and modifying RNA through temporary and ongoing treatment. CEO at ProQR Therapeutics, Daniel de Boer, discusses how they are advancing RNA therapies in retinal disorders.

More than 200 biopharma leaders acknowledge that out-of-pocket costs for individuals must be limited to sustain support for fiscal and patent policies key to advancing innovation.

An award made by the European Research Council allows Antonella Viola and her team at the University of Padova to be able to put funds into proof of concept work on using monoamine oxidase B inhibitors as novel drugs targeting NLRP3 inflammasome.

While not setting any records for the rapid approval of new drugs in 2019, FDA did speed a number of important new therapies to patients, writes Jill Wechsler.

The festivities marking the late 2019 holiday season in Europe have been spoiled by a disconcerting message about the declining efficiency of investments in pharmaceutical research, in the shape of a lengthy report from the European Union on industry’s innovative capacity.

The need for efficient and timely development of new treatments for multiple serious conditions will drive efforts to modernize clinical research in the coming year.

The European Union system for pharmacovigilance “is operating effectively and efficiently” concludes a report.

David Arthur, CEO of Salarius, discusses how companies like Salarius are incorporating endpoints into early-stage clinical trials that could offer a glimpse into efficacy.

Doug Manion, CEO of Kleo, explains how platform technology essentially involves the engineering of antibodies and biologics using linear process chemistry to enhance drug effectiveness.

How sharing patient data drives trial engagement and honors patient contributions to the research process.
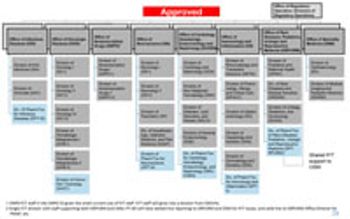
FDA’s Office of New Drugs restricting aims to improve scientific exchange and information sharing among review professionals.

Centralized monitoring offers sponsors and CROs the program-wide oversight they need to successfully develop the medications of the future, says CluePoints CEO, Francois Torche.

Do users actually want to use Excel? Explore the different viewpoints behind choosing SaaS or Excel.

The balance is being sought in Europe data governance between private protection and public benefit-but has not yet been achieved.

Dr. Riam Shammaa of INTELLiSTEM discusses his discoveries, how he plans to tackle clinical trial and therapy development challenges, and what the future development of cell-gene based cancer treatments look like.

Peter O’Donnell explores where the EU stands today in R&D pursuits for new antibacterial therapies.

Patrick Jordan, Chief Executive Officer of Mycovia, discusses the company's strategies on tackling Recurrent Vulvovaginal Candidiasis.

Europe’s additional monitoring scheme hasn't been a roaring success, according to a recent report.

While the future of the industry is still uncertain, a slew of uplifting ideas around innovation could help turn things around.

Seth Lederman, CEO of Tonix Pharmaceuticals, discusses their diverse research and development pipeline-including potential therapies for fibromyalgia, cocaine intoxication, and PTSD.

Cherry-picking a small group here and a dementia risk factor there won't reflect the way that dementia affects people from every walk of life, writes CEO & Founder of Savonix, Mylea Charvat.

A shift in emphasis in healthcare strategy could reduce attention and funding to therapy and impose tougher controls over research projects, or possible mean a boost for innovative healthcare. A look at where these trends may go.

After a court case rehashes the issue of excessive secrecy in trial data, drug firms maintain their objections over releasing data on their products.

CDER's Rare Disease Cures Accelerator initiative looks to foster a coordinated research approach and methods that can expedite development of drugs to treat some of the 7,000 rare diseases.

The time to stop hammering square pegs into round holes in terms of design strategy has come, as sponsors embrace an adaptive supply chain approach.

With a month left to join one of the 24 ERNs, Peter O'Donnell writes that this initiative is a welcomed demonstration of how cooperation can function in the common interest.

As a new industry survey shows, factoring in a generational cohort surfaces some provocative results related to key health indicators in clinical outsourcing relationships.
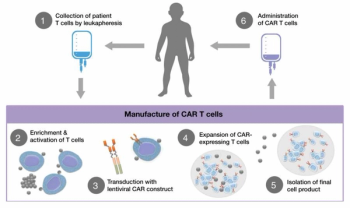
Executive Medical Director of Oncology for Premier Research, Emile Youssef, discusses crucial challenges that must be addressed in order to fulfill the promise of precision oncology.

BioXcel Therapeutics Executives, Vimal Mehta and Vince O’Neill, discuss how they are actively using artificial intelligence to discover advanced therapies in CNS and oncology.