
In this interview, Kevin Hudziak will expand on Lilly’s initiatives to change the face of clinical trials through patient engagement and education initiatives.

In this interview, Kevin Hudziak will expand on Lilly’s initiatives to change the face of clinical trials through patient engagement and education initiatives.

Peter O’Donnell weighs the varying views in Europe on the risks of going the adaptive pathways route for drug approval.

Lisa Henderson writes on patient participation and recruitment in clinical trials.

The rare disease community in Europe has come out fighting to defend its record-and its future-in the face of what it sees as growing threats to research.

By leveraging EHR data, the industry can transform how it conducts clinical research and delivers health care in the future.

FDA funding increased under Trump's budget for 2019 fiscal year while resource reductions take place for NIH.

The 10th anniversary of the Clinical Trials Transformative Initiative provided an opportunity for FDA officials to join with study sponsors and research experts to examine the policy achievements and plans for future efforts of improving clinical trials.

During the CROWN congress, there was discussion on CRO acquisition and consolidation, growing interest in the use of Blockchain technology in clinical trials, and new patient engagement methodologies.

In this interview, Jennifer Prichard, MD, Medical Monitor at Atlantic Research Group, and Hunter Walker, CTO at Atlantic Research Group will discuss challenges with existing medical monitoring processes, and how new technologies can help improve efficiency.

An industry shift has led toward more insourcing and in-house resources to run trials.

Peter O’Donnell looks at efforts in Europe to improve R&D communication and trust with investors and the public.

Lisa Henderson reports on the J.P. Morgan Healthcare conference and the balance of investment and science.

The race to build the new EMA location in time for its move is becoming a crucial topic.

In this interview, Dr. Michelle Longmire, CEO of Medable, will discuss her perspective on the advancements of digital health in clinical trials.

As the EMA celebrates its 2017 highlights, the new year brings deeper challenges for the agency-including preserving the credibility of its patient-first mission.

Kristy Galante, Director Process and Infrastructure of External Alliances at Janssen, recently spoke about a novel vendor oversight model at ExL’s 8th Clinical Quality Oversight Forum, and will expand on the model in this interview.

In this interview, Julie Dietrich will provide more information regarding this Clinical Research Access & Information Exchange Initiative and how it is expected to benefit patients and the industry.

In this interview, Christa Polidori, Clinical Trial Disclosure Manager at Bristol-Myers Squibb and a leader for the TransCelerate Clinical Research Access and Information Exchange Initiative, will discuss the TransCelerate proposal in greater detail.

2017 has been an exciting year for the clinical trials industry on many fronts, driven by new methods, regulation, and technology, but what is to come in 2018?

Mobile health devices are poised to transform medical care-and with it the medical device market.

In this interview, Austin Speier, VP of Emerging Technologies at Precision for Medicine, will elaborate on these draft guidance documents.

This article will explore the issues of a lack of data standards; focusing on an industry hackathon that analyzed data sets relating to rare diseases.

Sponsors should be aware of the significant implications the Addendum is likely to have on clinical trial planning, conduct, statistical analysis, and interpretation.

Sandy Chase focuses on the voice of the patient and their experience after not completing a clinical trial.

Investigators aim to increase the efficiency and consistency of FDA’s Bioresearch Monitoring program.

In this article, we will suggest an introspective method that can help clarify situational analysis and resolution.

With the literal toss of a coin officially sending the EMA to Amsterdam post-Brexit, it's worth reflecting on what might have been if the decision went differently.
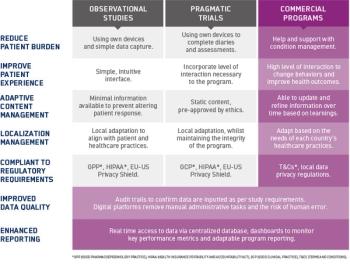
In this three-part blog series, we look at how electronic platforms can support and enhance data capture in several types of RWD programs: observational studies, pragmatic trials, and commercial programs.

The decision of where to move the EMA was determined by a coin toss.

Machine learning technology is finding it’s way into today’s marketplace, specifically within business processes, by driving insurmountable value for corporations within the life science industry, writes Adam Ross Miller.