
The European Union, looking to re-invent itself in the wake of Brexit, is considering withdrawing policy in the field of public health.

The European Union, looking to re-invent itself in the wake of Brexit, is considering withdrawing policy in the field of public health.

This survey on Endpoint Adjudication evaluates how this concept is impacting study design, uncovering operational challenges and determining outcomes.

With sixty million Americans and Europeans diagnosed with one or more of 7,000 defined rare diseases, the availability of treatments has been less than expected.

With the U.K. poised to begin the process of leaving the European Union soon, many questions remain as to the specifics of pharma regulation and the wide-ranging effects of this departure.

The Belgium-based EURORDIS symposium on rare diseases set out to realize dreams of accessible treatment for all, but the first day left much to be desired for this achievement.

The TMF Reference Model has released a survey for industry professionals designed to provide insight for both paper and electronic trial document management.

Pressure from the FDA and Congress has resulted in sponsors enrolling more women, elderly and ethnically diverse individuals in clinical studies.

The pharma industry needs an improved risk-based management approach to better handle the increased complexity of trials, to improve the quality of studies and to better adhere to new guidelines from regulatory agencies.

As pharma continues to struggle with designing studies that result in creating breakthrough medical therapies, biopharma sponsors focusing on CNS studies face similar challenges and risks.

Adopting eConsent provides patients with adequate information on studies they may choose to participate in, and thus improving the rate of patient satisfaction and retention.

FDA bioresearch monitoring staffs are being prompted to update and refine systems for targeting inspections to key study sites.

The latest version of the Prescription Drug User Fee Act has sponsors and FDA officials pressing for Congressional action regarding the program’s provisions accelerating the drug development and application review processes.

The war on cancer has seen significant strides made in the search for a cure, however, recent data suggests that the work is far from over.

Europe is issuing a significant update to its ethical guidelines governing health-related research involving humans.

The FDA has released a report that documents the value of large, confirmatory Phase III studies in ensuring the safety and efficacy of experimental medical products.

The European Union effort to forge more cooperation among its national and regional HTA bodies is still a work in progress, as the process has produced varying views on the subject.

The FDA’s Office of Regional Affairs will look to implement their much-anticipated Program Alignment initiative, thus reorganizing the FDA field force in 2017. This new program will alter bioresearch monitoring of clinical research operations.

The 21st Century Cures Act, passed in December, contains provisions that many have questioned and criticized. This review of those provisions shows that progress in this sector is being made.

A Brussels–based European Union conference on social rights set the stage for how access to healthcare should be widened with improved balance between innovation and affordability.
Nadir Benouali of MEDICODOSE Systems speaks on his experiences with investigational product nonadherence, its place in pharma and patient-centric ways to address it.

A technology-driven approach to oversight of vendors and sites can provide sponsors with timely and proactive solutions toward minimizing risks.

Finding the right clinical trial for yourself or others can be confusing and challenging. Matching services could provide potential relief, but only if aligned with both the physician and the patient.

A revised version of the Common Rule will remove the need for additional informed consent when studying biospecimen data of clinical trial participants.
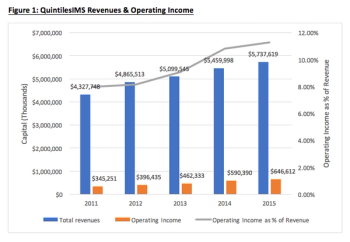
The CRO industry is experiencing exponential growth as a result of higher demand from the biopharma industry and an increased investment in R&D. This evaluation of QuintilesIMS attempts to provide some hints as to how this sector is advancing in the biopharma industry.
Roger DeRaad of Black Hills Cardiovascular Research and his staff discuss their experiences using IRT systems with 4G Clinical’s Kathleen Greenough. They offer insights into how sites, sponsors and technology vendors can cooperate to bring simplicity to clinical trials.

The standardization of trial metrics offers numerous benefits towards overseeing clinical trials, optimizing clinical operations and mitigating study risks. However, on a situational basis customized metrics may become necessary to address study-specific risks, uncover unknown issues and demonstrate results.

A prediction by QuintilesIMS consultants states that the industry can look forward to a historically large number and quality of new medicines emerging from the research and development pipeline over the next five years.

This article provides the necessary steps to advance past Phase II clinical trials on the way toward delivering an impactful drug to market.

The European Reference Network on Rare Respiratory Diseases-a grouping of specialists from more than 60 dispersed centers-has won approval of EU funding to maximize clinical trial efficiency in Europe.

European Parliament has voted through a resolution criticizing the performance of drug companies on pediatric medicines development. This resolution will punish drug companies who neglect to investigate possible pediatric applications of new medicines.