
Newest revelations suggest Risk Assessment Categorization Tools are now becoming therapeutic area-specific, as an oncology-based risk library is now surfacing.

Newest revelations suggest Risk Assessment Categorization Tools are now becoming therapeutic area-specific, as an oncology-based risk library is now surfacing.

BMS executive shares his perspectives on the current biomarker landscape, the role of translational medicine in accelerating this area of study, and how BMS is working to advance biomarker research across their R&D portfolio.

Jill Wechsler interviews FDA Commissioner Scott Gottlieb about his push for more efficient R&D to help lower drug prices.

Peter O’Donnell explores potential parallels of the U.S. “right-to-try” debate in Europe.

May includes a number of events to get more visibility for clinical trial awareness, and to offer it as a care option.
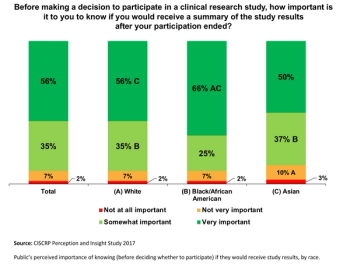
The third in a series of results from the Center for Information and Study on Clinical Research Participation’s (CISCRP) landmark 2017 Perceptions & Insights Study.

An additional light has been shone on the "right-to-try" debate in Europe by a strongly-worded statement from Belgium's feisty health minister, Maggie De Block.

The British government continues to offer guarantees and reassurances about the post-Brexit future of its life-sciences sector despite the obvious failure so far to get even close to negotiating a withdrawal agreement with the EU.

Recent legislation authorizes further assessment of FDA eligibility policies and of NIH research standards.

Agile Product Development Methodology holds great promise for use in Adaptive Clinical Study Development.
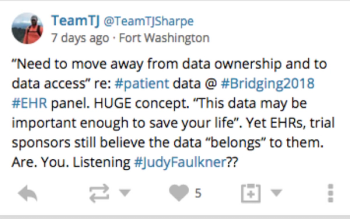
Lisa Henderson talks about the topic of "who owns a patient's data" as discussed at the Bridging Clinical and Healthcare Conference 2018.

In this interview, Dr. David Lee Scher adds his perspective on how digital health is changing in both healthcare and clinical trial settings.

The EU General Data Protection Regulation is the most important change in data privacy regulation in 20 years.

The topic of clinical trial quality and compliance continues to evolve in clinical operations, based on discussions at ExL’s 9th Proactive GCP Compliance Conference.

Debunking the six most common myths regarding electronic informed consent.

Jill Wechsler on the tug of war in accelerating orphan drug development.

Relocating the European Medicines Agency was always going to be hard-but no-one ever expected it to degenerate into farce.

Lisa Henderson discusses randomized clinical trials with active and non-active placebo treatments.

Though digital technology has improved the R&D process, when it comes to important clinical benefits and outcomes, modern biotech products have rarely shown the advantages of older generations of drugs.
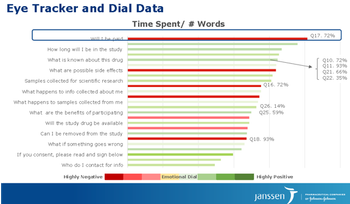
At ExL’s CROWN Congress, Cassandra Smith, Associate Director, Investigator & Patient Engagement at Janssen, discussed results from a study they conducted with patients on consent content modification.

Renewed attempts in the United States to win legislative approval for the right-to-try concept have again focused attention on that inevitably grey area between hoping a new medicine is going to be effective and demonstrating that it can be.

Building upon the usage of technology in health care delivery, integrating technology into clinical research as a care option will be a game changer in achieving greater participation in clinical research and decreasing the overall time for trial completion.

To encourage experimentation with more complex clinical trial strategies, FDA plans to launch a pilot program to assess complex studies proposed by sponsors.

Education Falsification has more consequences than not having a degree.

This article will analyze industry clinical trial initiatives and investigate strategies on how to improve impact on people.

Relocating the European Medicines Agency was always going to be hard-but no-one ever expected it to degenerate into farce.

An overview of all the drug approvals that were secured in 2017.

The use of Learning Health Systems in order to advance clinical research.

Under pressure to meet tight deadlines for reviewing and approving a growing volume of applications for new drugs, generics, and medical products, FDA is rejecting submissions that are incomplete or unsatisfactory right from the start.
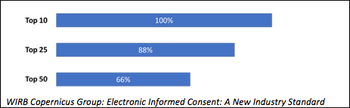
Debunking the six most common myths regarding electronic informed consent.