
New actions underway seek to find a consensus on the role of HTAs in the decision-making chain, including with clinical trials.

New actions underway seek to find a consensus on the role of HTAs in the decision-making chain, including with clinical trials.

Industry professionals are searching for new technologies to support their trials following approval of an NIH policy supporting single IRB practices. Transparent and auditable systems can offer stakeholders the ability to more effectively monitor and report regulatory responsibilities.

Gene-editing technology has the ability to give oncology researchers an effective treatment option in the fight against cancer. CRISPR-Cas9 is such a technology that is currently being used to study genes in cancer cells.
Most people in our poll said it was a service, but others disagreed. Find out more in this follow-up from Michael Howley PA-C, PhD and Associate Clinical Professor, Department of Marketing at the LeBow School of Business at Drexel University.

European Biopharmaceutical Enterprises (EBE) warns that Europe’s new drug ideas are going to waste due to a lack of innovative support in the sector. The EBE has offered several requests to stimulate investment, but attracting support from member state governments and the European Commission will be no easy task.

Functional dyspepsia is noted globally as being a common condition that currently has no specific therapy despite years of research. This condition is in need of an operational definition so that drug development and treatment trials can move forward with a more definitive trial design.

Technology innovations have been introduced into the clinical trials space that have the ability to change the trial experience for all involved. Egg’s TRIAL 360 platform is no exception, as its goal is to provide a networking platform to keep investigators and study stakeholders engaged in the trial.

The FDA’s objectives regarding pediatric labeling have been misunderstood due to a confusing history on the matter. Data extrapolation can leverage an avenue for providing more comprehensive labeling for pediatric drugs.

Antimicrobial resistance presents a challenge on a global scale that has received attention from the United Nations General Assembly among other governing bodies. Research and development for new antimicrobials and alternative medicines is needed to combat such a threat.

A critical challenge that clinical trial researchers face is patient drop out. Why patients fail to complete studies can be attributed to a variety of reasons, and delays in compensation can often be one of them.

After months of debate, clinical research activity regulated or funded by the government must adhere to revised guidelines regarding transparency. A final rule published by the Department of Health and Human Services states that all beyond Phase I FDA regulated and NIH funded clinical trials must comply with the new requirements.

The clinical trials space has shifted to a patient friendly, fast and easy process thanks to technological improvements, despite some gaps in the existing model. A Clinical Research Care Option (CRCO) can fill these gaps by fostering a more inclusive clinical research environment.

Patients of gastroesophageal reflux disease (GERD) that have been frequently diagnosed, or misdiagnosed, have been dissatisfied with the available treatment. To resolve this issue, industry players must define meaningful endpoints for future trials, incorporate patient reported outcomes, and apply biomarkers.

Cancer therapy has seen progress - not groundbreaking, but improving thanks to the scientific and technological tools at our disposal. This fall offers industry events and trial evaluations that aim to continue this progressive trend toward a breakthrough.

Technology platforms are today continually being adapted into the clinical trial life cycle with the promise of efficiency and reduced risk. CRF Health believes its combined eCOA/eConsent solution could improve the flow of documentation between investigators and sponsors.

With registries gaining steam as a potential European policy agenda, the EMA plans to incorporate them into the healthcare community. This movement aims to solve the issues of getting the right drugs to the right patients at the right time, and at something like the right cost.
Gastroparesis patients face a series of symptoms for which there is currently a significant void in treatment. With complications and questions aplenty, answers are in high demand as physicians search for suitable medicines to combat this disease.

Conducting observational studies is becoming a vital part of the clinical trial process, but research teams often struggle to operationalize these more creative and non-traditional study methodologies.

Cellular therapies offer potential to improve medicine and fill the needs of patients with few treatment options, though these products must be stored at cryogenic temperatures. Incorporating cold chain logistics into your clinical trial can prove beneficial in making these products available to patients.

Investigational sites’ readiness to support new monitoring initiatives such as risk-based monitoring and centralized monitoring will dictate their success. Here are key aspects that sponsors and CROs should establish with investigational sites while implementing these new initiatives.

Eosinophilic esophagitis (EoE) is a rare esophageal condition that currently has no FDA-approved medications resulting in major gaps in treatment. Therefore, the need for better options and opportunities for drug development is apparent.

The way the Open Payments system is couching investigator transactions is questionable and confusing.

EU Research Commissioner Carlos Moedas says all data generated under the $350 million Horizon 2020 projects will be open access. This supports the shift of greater cooperation in research and a new era of global and open science.

Despite the increasing number of clinical trials in the United States, still participation remains at an all-time low. You can make a difference with participation in INC Research and CISCRP’s “Inspiring Hope’ Ideathon by submitting ideas to increase clinical research awareness.

A look at the EMA’s proposed guidance revisions for first-in-human studies.

The European Medicines Agency is proposing changes to the current guidelines on first-on-human clinical trials to improve risk-based strategies.

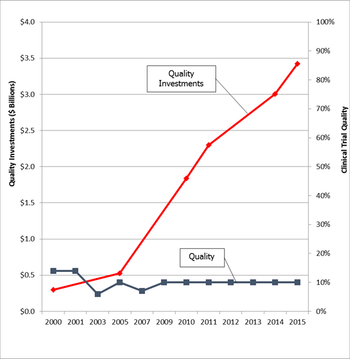
ClinCapture hosts a panel discussion with experts from CROs and sponsor companies to discuss conducting quality clinical trials in a cost effective manner.

Continuous Glucose Monitoring (CGM) devices have the ability to replace the traditional finger-prick to measure glucose levels in a patient’s blood. Quintiles’ device expert, Sam Osman, explains CGM and how the FDA decision could affect clinical trials.

The challenges of patient recruiting must be addressed if medical breakthroughs are to be made. Education, simplicity and patient feedback could go a long way in improving the recruitment process.

While Europe is feeling the effects of the recent UK Brexit vote, the European Medicines Agency is continuing onward with business as usual despite questions over its location and the future of its employees.